CLEVELAND & ATHENS, Greece--(BUSINESS WIRE)--Juventas Therapeutics, Inc.,a private clinical-stage company developing a drug-based approach to regenerative medicine, today presented top-line interim data from a Phase II study evaluating safety and efficacy of JVS-100 in patients with symptomatic ischemic cardiomyopathy at the European Society of Cardiology- Heart Failure Congress in Athens, Greece.
In an oral presentation accepted as a late-breaking clinical trial, Marc Penn, M.D., Ph.D., Founder and Chief Medical Officer for Juventas and Director of Cardiovascular Research at Summa Health System, Akron, Ohio, presented the interim data from the STOP-HF trial that shows JVS-100, a non-viral gene therapy expressing stromal cell-derived factor 1 (SDF-1), improves cardiac function in patients with advanced heart failure more than a decade after having a heart attack. SDF-1 has been shown in previous studies to promote tissue repair through activating an endogenous stem cell repair pathway.
“This is an important milestone in our development of JVS-100 as a first-in-class therapy for heart failure,” Penn said. “We’ve observed consistent dose-dependent responses across multiple objective measures that provide strong support for JVS-100 bioactivity in patients with a history of a heart attack that have progressed to symptomatic heart failure.”
At the time of the interim analysis, 93 ischemic symptomatic cardiomyopathy patients with prior history of a heart attack were randomized on a 1:1:1 ratio to receive a single treatment of either 15 mg or 30 mg of JVS-100 or matching placebo. Objective and subjective measures of clinical status are being assessed in all patients at 120 days (4 months), and 360 days (12 months) post-injection.
The study demonstrated that JVS-100 was well tolerated in all patients with no serious side effects. To date, JVS-100 has been safely delivered to more than 150 patients across multiple clinical trials.
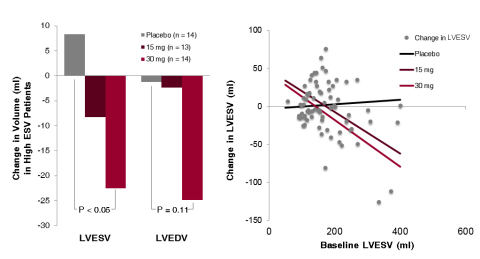
STOP-HF Interim Results
Interim top-line four-month efficacy
data demonstrated dose-dependent improvements in echocardiographic
parameters and biomarkers for patients having received JVS-100.
Specifically, a subpopulation of high risk patients who received the
high dose of JVS-100 (30 mg) demonstrated improvements relative to
placebo in left ventricular end systolic volume (LVESV) p<0.05, left
ventricular end diastolic volume (LVEDV) p=0.11, left ventricular
ejection fraction (LVEF) p=0.23 and NTproBNP levels (a simple blood test
that predicts the risk of worsening heart failure in patients with known
cardiovascular disease) p=0.12.
“The structural improvements to the heart in STOP-HF patients observed at four months in response to JVS-100 are compelling, especially in patients with more advanced disease, who represent a high risk heart failure population,” said Eugene Chung, M.D., FACC, co-principal investigator for the trial and Director, Advanced Heart Failure Program at The Christ Hospital. “The data suggests that the SDF-1 pathway induces left ventricular remodeling leading to trends of clinically meaningful volume reductions in patients. The literature has shown that improved cardiac function correlates with improved patient outcomes. The clinically meaningful changes we are observing in end systolic volumes and ejection fraction warrant further clinical investigation of JVS-100 as a potential therapy for heart failure.”
Study drug was administered directly to the heart via the BioCardia Helical Infusion Catheter (HIC), which is being developed by BioCardia, Inc., of San Carlos, Calif. The HIC allows for percutaneous delivery of biologics to treat cardiovascular disease and provides a new therapeutic option for patients with heart failure and chronic myocardial ischemia.
“The HIC continues to demonstrate a strong safety profile for delivery of biologics to the heart,” said Warren Sherman, M.D., co-principal investigator for the trial and Director, Stem Cell Research and Regenerative Medicine, Center for Interventional Vascular Therapy, Columbia University Medical Center. “All patients were successfully dosed with no unanticipated serious adverse events related to the device or procedure.”
About JVS-100
JVS-100, is a non-viral plasmid that encodes
for stromal cell-derived factor-1 (SDF-1). SDF-1 promotes cardiac
function and tissue repair following a heart attack through activation
of the body’s natural stem-cell based regenerative repair processes.
JVS-100 is the subject of active clinical programs in heart failure (HF)
and critical limb ischemia (CLI). JVS-100 is the focus of an on-going
Phase II blinded, randomized, placebo-controlled trial, RETRO-HF, which
is currently enrolling patients similar to those enrolled in STOP-HF
with delivery via coronary sinus infusion.
About the STOP-HF Phase II Trial
STOP-HF is an exploratory
Phase II study of 93-patients in a double-blind, randomized,
placebo-controlled trial being conducted at 15 centers in the United
States to evaluate safety and efficacy of a single treatment of JVS-100,
a plasmid stromal cell-derived factor 1 (SDF-1) delivered via injection
to the endocardium and myocardium of patients with ischemic heart
failure.
Patients enrolled in STOP-HF have a prior history of a heart attack and years later developed symptomatic heart failure as defined by an ejection fraction (a measurement of how well your heart is pumping) less than or equal to 40 percent and poor quality of life and exercise tolerance as measured by the Minnesota Living with Heart Failure Questionnaire (MLWHQ) and six minute walk distance (6MWD), respectively.
Endpoints of STOP-HF include objective echocardiographic parameters of left ventricular remodeling and biomarkers as well as subjective clinical measures of clinical status in heart failure patients.
About Juventas Therapeutics, Inc.
Juventas Therapeutics,
Inc., headquartered in Cleveland, Ohio, is a privately held
clinical-stage biotechnology company developing a pipeline of
regenerative therapies to treat life–threatening diseases. Founded in
2007 with an exclusive license from Cleveland Clinic, Juventas has
transitioned its therapeutic platform from concept to through mid-stage
clinical trials for treatment of heart failure and critical limb
ischemia. Investors include Triathlon Medical Venture Partners, New
Science Ventures, Fletcher Spaght Ventures, Takeda Ventures, Venture
Investors, Early Stage Partners, Reservoir Venture Partners, and
Cleveland Clinic. The Company has also received financial support
through the Ohio Third Frontier-funded Global Cardiovascular Innovation
Center and Center for Stem Cell & Regenerative Medicine. For more
information, please visit www.juventasinc.com.