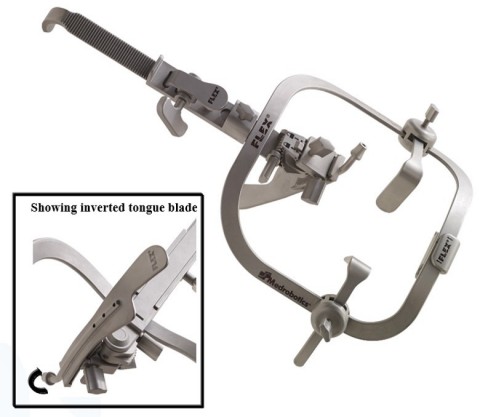
RAYNHAM, Mass.--(BUSINESS WIRE)--Medrobotics Corp., a surgical products company with core competencies in medical robotics, today announced the market release of its Flex Retractor® in the U.S. and Europe.
The Flex® Retractor is lightweight and easy to handle and position. It has been designed to facilitate exceptional exposure in advanced transoral surgeries, including robotic and laser microsurgery procedures. Medrobotics has incorporated an industry first axial tongue blade rotation capability, which allows surgeons to create unprecedented exposure in certain procedures involving the base of tongue.
Dr. Marshall Strome, M.D., Professor and Chairman Emeritus, Cleveland Clinic Head and Neck Institute and Co-Chair of Medrobotics Medical Advisory Board, said, “The Flex® Retractor is a significant advance for transoral surgery. With its unique adjustability and ease of use, the Flex® Retractor offers head and neck surgeons the confidence that they can efficiently achieve the exposure that they need for advanced transoral procedures.”
The Flex® Retractor is the latest example of innovative and yet affordable technology for minimally invasive surgery from Medrobotics, the manufacturer of the Flex® System. Minimally invasive surgery has demonstrated advantages for patients and providers compared to traditional open procedures, decreasing hospital stays and recovery times. The Flex® System was designed to provide an affordable, easy-to-use computer-assisted surgical platform for hospitals and surgeons seeking to provide minimally-invasive treatment options to the broadest number of patients.
Medrobotics received the CE mark for the Flex® Retractor which permits distribution in the European Union. The Company has met all applicable FDA requirements to distribute the Flex® Retractor in the U.S. where it is a class I device and exempt from FDA’s premarket notification process.
About Medrobotics
Medrobotics Corporation (www.Medrobotics.com) is a privately-held company headquartered in Raynham, Massachusetts that manufactures and markets the Flex® System, a computer-assisted platform that provides surgeons with single-site access and visualization of hard-to-reach anatomical locations. The Company’s vision is to provide more patients with access to minimally invasive surgery. Medrobotics recently received the CE mark for its Flex® System, which is available on a limited basis in Europe. The Flex® System is not currently cleared for sale in the U.S.