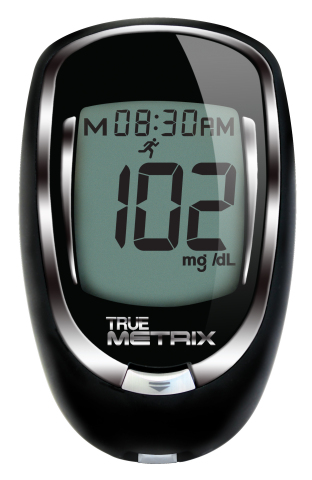
FORT LAUDERDALE, Fla.--(BUSINESS WIRE)--Nipro Diagnostics, Inc., today announced that the U.S. Food and Drug Administration (FDA) has cleared its TRUE METRIX™ Self-Monitoring blood glucose system and the TRUE METRIX™ PRO Professional Monitoring blood glucose system.
As the diabetes store-brand leader, and largest U.S. manufacturer of diabetes products for the store brand market, Nipro Diagnostics made the announcement at the National Association of Chain Drug Stores (NACDS) Total Store Expo in Boston where more than 250 retail companies, representing over 145,000 retail outlets, were present.
Nipro Diagnostics conducted a clinical study in the United States for submission to the FDA which was designed to assess the accuracy of the TRUE METRIX Self-Monitoring blood glucose system and the TRUE METRIX PRO Professional Monitoring blood glucose system when tested by lay users and healthcare professionals, respectively. As well, the clinical data demonstrated that the two meter systems exceeded the minimum accuracy criteria for the new, more stringent ISO 15197:2013 performance requirements for accuracy.
TRUE METRIX and TRUE METRIX PRO achieve a level of performance driven by science, research and technological advancements. The meter, a complex algorithm, test strip chemistry and electrodes, work together as part of the TRUE METRIX system to produce accurate results. Featuring TRIPLE SENSE TECHNOLOGY™, the systems detect, analyze and correct for environmental and physiological variability, including hematocrit and temperature, resulting in precise, proven accuracy.
The meters feature pre- and post-meal tags that enable patients to mark blood glucose results to better see how food affects blood glucose. Additionally, the products allow patients to tie notable events such as exercise, medication, sickness, as well as other occasions, to blood glucose readings in order to determine patterns and trends, resulting in better decision making for diabetes management.
Manufacturing of the proprietary new blood glucose sensor technology required significant capital investment, which will substantially expand manufacturing capabilities at Nipro Diagnostics’ Fort Lauderdale, Florida facility where research and development, engineering and test strip manufacturing take place for all of the TRUE branded blood glucose products.
“With this investment, Nipro Diagnostics is building on its more than 25-year heritage of manufacturing blood glucose test strips in the United States. As we invest further in the U.S., this expansion will help us meet an ever-growing demand for blood glucose test strips as the number of people with diabetes is expected to continue to increase. Six consecutive years of Nipro Diagnostics’ double-digit test strip growth has brought the need for sizeable manufacturing expansion, as demand for quality/value healthcare solutions continues to grow,” said Scott Verner, CEO and President of Nipro Diagnostics.
The high-speed, high-accuracy manufacturing line will employ the latest innovative technologies including precision lasers that ablate electrodes on the test strips, a machine vision system that inspects over 100,000 characteristics on every test strip, and an advanced, ultra high definition, state-of-the-art communications system that allows 100 percent strip quality assurance. This advanced manufacturing process delivers a high-quality, cost effective product with proven performance.
“The launch of TRUE METRIX and TRUE METRIX PRO strengthen Nipro Diagnostics’ leadership position in blood glucose monitoring and join a quickly expanding ecosystem of high-quality, high-value healthcare management products including nutritional, OTC healthcare and dietary supplements specifically designed to meet the health and wellness needs of the diabetes patient population. TRUE METRIX is a valued addition to our growing portfolio of healthcare management products,” stated Verner.
“It is essential as the store brand leader in diabetes that we continue to innovate from both a technology and a business perspective. The evolving payer landscape, driven by demands of increased clinical performance and economic accountability, has created a market energized in disruption. The TRUE METRIX blood glucose monitoring platform reinforces our commitment to offer an innovative, affordable solution for diabetes patients to lead healthier lives,” Verner continued.
TRUE METRIX will be made available through Retail, Mail and Distribution channels beginning Q4, 2014 under partner brands or under Nipro Diagnostics’ own TRUE brand of products. Maintaining its core value proposition as the quality/value manufacturer in the diabetes category, products will be available at a value price point. Nipro Diagnostics will work with payors and providers to add this platform to the millions of exclusive and preferred lives currently on formulary.
About Nipro Diagnostics, Inc.: Based in Fort Lauderdale, Florida, Nipro Diagnostics, Inc. is a leading developer, manufacturer and marketer of diabetes monitoring and management products. The company offers a portfolio of high-quality blood glucose monitoring systems and diabetes management products available around the world. Nipro Diagnostics is the exclusive supplier of blood glucose monitoring systems, co-branded under the TRUE name, to the world’s leading pharmacies, distributors and mail service providers. For more information, please visit: www.niprodiagnostics.com
NICO-3393 ©08/2014 Nipro Diagnostics, Inc. TRUE METRIX and TRIPLE SENSE TECHNOLOGY are trademarks of Nipro Diagnostics, Inc.