Analysis on Impact of COVID-19- Global Therapeutic Vaccines Market 2020-2024 | Evolving Opportunities with Aimmune Therapeutics Inc. and Immune Response BioPharma Inc.| Technavio
Analysis on Impact of COVID-19- Global Therapeutic Vaccines Market 2020-2024 | Evolving Opportunities with Aimmune Therapeutics Inc. and Immune Response BioPharma Inc.| Technavio
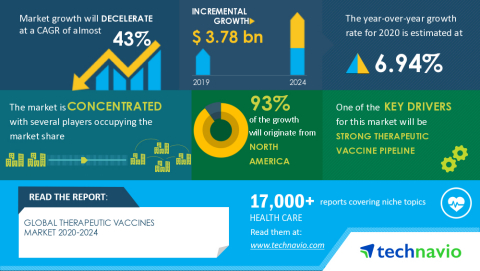
LONDON--(BUSINESS WIRE)--The global therapeutic vaccines market size is expected to grow by USD 3.78 billion as per Technavio. This marks a significant market growth compared to the 2019 growth estimates due to the impact of the COVID-19 pandemic in the first half of 2020. However, the market is expected to decelerate at a CAGR of almost 43% during the forecast period. Request Free Sample Report on COVID-19 Impacts
Read the 120-page report with TOC on "Therapeutic Vaccines Market Analysis Report by Type (Cancer vaccines, Infectious diseases vaccines, Neurological diseases vaccines, Autoimmune diseases vaccines, and Other diseases vaccines) and Geography (North America, Europe, Asia, and ROW), and the Segment Forecasts, 2020-2024."
https://www.technavio.com/report/therapeutic-vaccines-market-industry-analysis
The market is driven by the presence of a strong pipeline. In addition, the increase in R&D activities related to novel therapeutic vaccines is anticipated to boost the growth of the therapeutic vaccines market.
The increase in the prevalence of infectious diseases and chronic diseases has driven the demand for therapeutic vaccines. The market has witnessed the development of various therapeutic vaccines by top vendors in the market. For instance, Nexvax2, a therapeutic vaccine developed by ImmusanT for the treatment of celiac disease, is currently in the Phase II stage of clinical trials. In January 2019, the US FDA granted Fast Track designation to Nexvax2. Currently, no US FDA-approved treatment is available for the treatment of celiac disease. Therefore, the granting of Fast Track designation will lead to accelerated development of Nexvax2. Similarly, very few products are available in the market for the treatment of human papillomavirus infections. Inovio Pharmaceuticals developed a potential immune-based therapeutic vaccine known as VGX-3100 to treat precancers in the cervix, which eliminates both the lesion and the underlying human papillomavirus infections in women. The therapeutic vaccine is currently in Phase III stage of clinical trials.
Buy 1 Technavio report and get the second for 50% off. Buy 2 Technavio reports and get the third for free.
View market snapshot before purchasing
Major Five Therapeutic Vaccines Companies:
Aimmune Therapeutics Inc.
Aimmune Therapeutics Inc. operates through one reportable segment, which focuses on developing proprietary product candidates for the treatment of peanut and other food allergies. The company’s key offerings include AR101, which is an oral immunotherapy drug for the treatment of patients with peanut allergy, currently under Phase III of clinical trials.
Immune Response BioPharma Inc.
Headquartered in the US, Immune Response BioPharma Inc. offers various therapeutic vaccines, such as RAVAX, NeuroVax, Zorcell, and RemuneX. RAVAX is a combination vaccine under Phase III clinical trials for treatment of patients with an HIV-1infections. NeuroVax is a therapeutic vaccine under Phase II of clinical trials for treatment of patients with multiple sclerosis. Zorcell is a therapeutic vaccine under Phase II of clinical trials for the treatment of psoriasis. RemuneX is a combination vaccine under Phase III clinical trials for treatment of HIV-1 infections.
Inovio Pharmaceuticals Inc.
Inovio Pharmaceuticals Inc. focuses on the discovery, development, and commercialization of DNA immunotherapies for cancer and infectious diseases. The company offers VGX-3100, which is a therapeutic DNA vaccine under Phase III clinical trials for treatment of patients with cervical dysplasia.
Novartis AG
Novartis AG operates its business through three segments, which include Innovative medicines, Sandoz, and Alcon. The company offers CAD106, which is a therapeutic vaccine under Phase II/III clinical trials for treatment of patients with Alzheimer's disease.
Sanpower Group Co. Ltd.
Sanpower Group Co. Ltd. is headquartered in the China. The company offers PROVENGE, which is a cell-based cancer immunotherapy for prostate cancer.
Register for a free trial today and gain instant access to 17,000+ market research reports.
Technavio's SUBSCRIPTION platform
Therapeutic Vaccines Type Outlook (Revenue, USD bn, 2020-2024)
- Cancer vaccines - size and forecast 2019-2024
- Infectious diseases vaccines - size and forecast 2019-2024
- Neurological diseases vaccines - size and forecast 2019-2024
- Autoimmune diseases vaccines - size and forecast 2019-2024
- Other diseases vaccines - size and forecast 2019-2024
Therapeutic Vaccines Regional Outlook (Revenue, USD bn, 2020-2024)
- North America - size and forecast 2019-2024
- Europe - size and forecast 2019-2024
- Asia - size and forecast 2019-2024
- ROW - size and forecast 2019-2024
Technavio’s sample reports are free of charge and contain multiple sections of the report, such as the market size and forecast, drivers, challenges, trends, and more. Request a free sample report
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio’s report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio’s comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: media@technavio.com
Website: www.technavio.com/