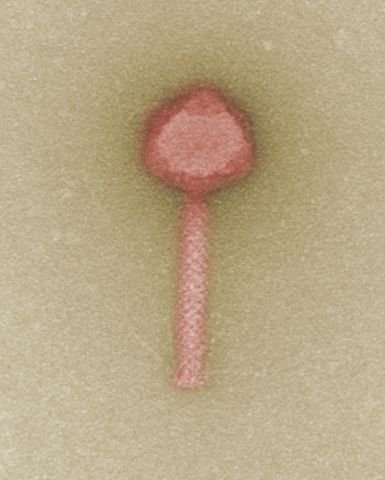
HANNOVER, Germany--(BUSINESS WIRE)--Bacteria worldwide keep developing new resistances to antibiotics. Alternative therapies are urgently needed to meet this challenge. To this end, the Fraunhofer ITEM, Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Charité – Universitätsmedizin Berlin, and Charité Research Organisation have teamed up and initiated the project “Phage4Cure”. The goal is to establish bacteriophages as an approved drug for treating bacterial infections. The German Federal Ministry of Education and Research is funding this project with almost four million euros over a period of three years.
The aim of the German research project “Phage4Cure” is to establish bacteriophages as an approved drug in the fight against infection. Bacteriophages are viruses that highly specifically recognize and bind to a certain type of bacteria, eventually causing their destruction. In eastern Europe in particular, phages have been successfully used for decades already as an alternative or complementary treatment to traditional antibiotic therapy. In the European Union, however, they have not yet been approved as drugs. This is due, among other reasons, to missing quality standards for bacteriophage production, a sine qua non for drug approval by the authorities. In addition, systematic clinical trials first have to be performed to demonstrate the safety, tolerability, and efficacy of treatment with phages. This is exactly the aim the project partners are now going for.
In the project “Phage4Cure”, the four partners with their special know-how are each working on a different aspect of their common topic. The Leibniz Institute DSMZ working group under Dr. Christine Rohde will identify bacteriophages targeting Pseudomonas aeruginosa, including genetic characterization. At Fraunhofer ITEM, the team under Dr. Holger Ziehr will develop a so-called platform manufacturing process for phage-based therapeutic agents – a manufacturing process that can subsequently be used for other phages as well. In addition, the Fraunhofer ITEM scientists will perform preclinical testing.
Further preclinical tests will be conducted at Charité – Universitätsmedizin Berlin by scientists from the team of Professor Martin Witzenrath. He is also involved in the design, planning, and performance of the required clinical trials. The research unit of Charité Research Organization will perform the clinical trial, besides providing organizational and regulatory assistance for the entire project, keeping close contact with supervisory drug agencies, and taking care of data management, statistics, and preparation of the clinical study report.
Further information: https://www.item.fraunhofer.de/en/press-and-media/press-releases/pm-fraunhofer-item-phage4cure.html