NEW HYDE PARK, N.Y.--(BUSINESS WIRE)--Patients seeking treatment for opioid and substance use disorders will now have access to an innovative FDA-authorized treatment option – prescription digital therapeutics (PDTs) – thanks to a collaboration between Northwell Health and Pear Therapeutics, Inc.
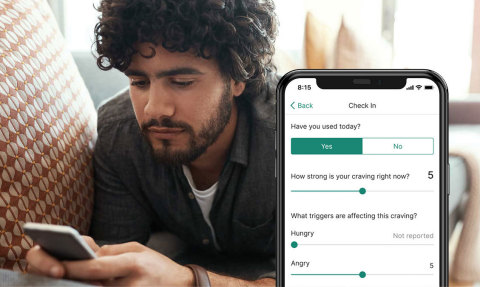
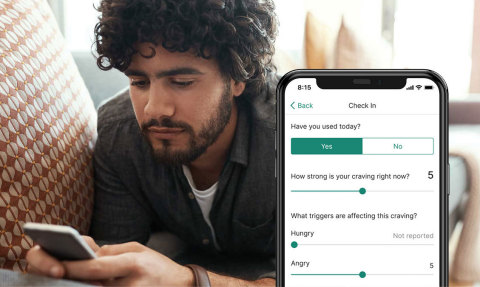
Northwell will be providing digital care to patients by offering access to Pear’s reSET® and reSET-O® PDTs through the substance abuse treatment programs offered through Zucker Hillside Hospital, Northwell’s stand-alone behavioral health facility.
“We are witnessing a substantial increase in substance misuse and opioid overdose death as a result of the social isolation, anxiety and stress related to COVID-19,” said Bruce Goldman, LCSW, director, Behavioral Health at Northwell Health. “Northwell Health has successfully transformed our ambulatory substance use treatment services to a telehealth model. To augment our treatment services, we have made available to our patients this FDA-authorized prescription digital therapeutic proven to increase the retention of patients in outpatient treatment programs.”
PDTs are a new therapeutic class that uses software to directly treat disease. However, unlike traditional medicines, PDTs are designed to collect real world data for use by prescribing clinicians and for population health management.
Noting that feedback from both patients and clinicians has already been positive, Mr. Goldman added, “We are so pleased that our clinicians can now prescribe these applications to support patients on their recovery journey.”
Because substance and opioid use disorders are chronic, treatable diseases, it is imperative that patients seek out an ongoing treatment program. Staying engaged in these programs is critical for people on their recovery journey. reSET and reSET-O will give patients a discreet, 24/7, evidence-based tool to complement their remote or in-office addiction therapy provided by their therapist.
reSET and reSET-O are the first two PDTs to receive market authorization to treat disease from the FDA. Both products have been tested in real world use and randomized controlled trials, with results published in peer-reviewed medical journals. Both products, which complement outpatient counseling, provide patients with algorithm-driven cognitive behavioral therapy, fluency training, and contingency management, while therapists can access this data to inform in-office and tele-visits.
“Pear is pleased to work with Northwell to bring suitable patients the latest in evidence-based substance and opioid use treatment options,” said Julia Strandberg, Chief Commercial Officer at Pear Therapeutics. “We believe the use of prescription digital therapeutics can provide patients with innovative and convenient care through their mobile devices to complement Northwell’s ambulatory substance use treatment services.”
If you or someone you know is interested in these services you can reach Zucker Hillside Hospital Addiction Services at 718-470-4289.
About Northwell Health
Northwell Health is New York State’s largest health care provider and private employer, with 23 hospitals, 830 outpatient facilities and more than 16,600 affiliated physicians. We care for over two million people annually in the New York metro area and beyond, thanks to philanthropic support from our communities. Our 76,000 employees – 18,900 nurses and 4,800 employed doctors, including members of Northwell Health Physician Partners – are working to change health care for the better. We’re making breakthroughs in medicine at the Feinstein Institutes for Medical Research. We're training the next generation of medical professionals at the visionary Donald and Barbara Zucker School of Medicine at Hofstra/Northwell and the Hofstra Northwell School of Nursing and Physician Assistant Studies. For information on our more than 100 medical specialties, visit Northwell.edu and follow us @NorthwellHealth on Facebook, Twitter, Instagram and LinkedIn.
About Pear Therapeutics
Pear Therapeutics is the leader in prescription digital therapeutics, or PDTs. Pear aims to redefine medicine by discovering, developing, and delivering clinically validated software-based therapeutics to provide better outcomes for patients, smarter engagement and tracking tools for clinicians, and cost-effective solutions for payers. Pear has a pipeline of products and product candidates across therapeutic areas, including the first three PDTs with disease treatment claims from FDA. Pear’s lead product, reSET®, for the treatment of substance use disorder, was the first PDT to receive marketing authorization from FDA to treat disease. Pear’s second product, reSET-O®, for the treatment of opioid use disorder, was the first PDT to receive Breakthrough Designation. Pear’s third product, Somryst® for the treatment of chronic insomnia, was the first PDT submitted through FDA’s traditional 510(k) pathway while simultaneously reviewed through FDA’s Software Precertification Pilot Program. For more information, visit Pear at www.peartherapeutics.com.
reSET Important Safety Information
Indications for Use
reSET is intended to provide cognitive behavioral therapy, as an adjunct to a contingency management system, for patients 18 years of age and older, who are currently enrolled in outpatient treatment under the supervision of a clinician. reSET is indicated as a 12-week (90 day) prescription-only treatment for patients with substance use disorder (SUD), who are not currently on opioid replacement therapy, who do not abuse alcohol solely, or who do not abuse opioids as their primary substance of abuse.
It is intended to increase abstinence from a patient’s substances of abuse during treatment, and increase retention in the outpatient treatment program.
Important Safety Information:
Warnings: reSET is intended for patients whose primary language is English and whose reading level is at the 7th grade level or above and who have access to an Android/iOS tablet or smartphone.
reSET is intended only for patients who own a smartphone and are familiar with use of smartphone apps (applications).
Clinicians should not use reSET to communicate with their patients about emergency medical issues. Patients should be clearly instructed not to use reSET to communicate to their clinician any urgent or emergent information. In case of an emergency, patients should dial 911 or go to the nearest emergency room.
reSET is not intended to be used as a stand- alone therapy for Substance Use Disorder (SUD). reSET does not represent a substitution for a patient’s medication. Patients should continue to take their medications as directed by their healthcare provider.
reSET should not be used by individuals outside active enrollment in a SUD treatment program. It should only be used as an adjunct to face-to-face counseling and contingency management. reSET is not intended to reduce the amount of face-to-face clinician time.
The long-term benefit of treatment with reSET on abstinence has not been evaluated in studies lasting beyond 12-weeks in the SUD population. The ability of reSET to prevent potential relapse after treatment discontinuation has not been studied.
The effectiveness of reSET has not been demonstrated in patients currently reporting opioids as their primary substance of abuse.
This Press Release does not include all the information needed to use reSET safely and effectively. Please see the Clinician Brief Summary for reSET for more information.
reSET-O Important Safety Information
Indications for Use
reSET-O is intended to increase retention of patients with Opioid Use Disorder (OUD) in outpatient treatment by providing cognitive behavioral therapy, as an adjunct to outpatient treatment that includes transmucosal buprenorphine and contingency management, for patients 18 years or older who are currently under the supervision of a clinician. reSET-O is indicated as a prescription-only prescription digital therapeutic.
Important Safety Information:
Warnings: reSET-O is intended for patients whose primary language is English and who have access to an Android/iOS tablet or smartphone. reSET-O is intended only for patients who own a smartphone and are familiar with use of smartphone apps (applications).
reSET-O should not be used by individuals outside active OUD treatment. It is not intended to replace treatment by you, the patient’s medical provider. It should be used as an adjunct to clinician treatment, buprenorphine treatment and contingency management.
reSET-O is not intended to be used as a stand-alone therapy for Opioid Use Disorder (OUD). reSET-O does not represent a substitution for a patient’s medication. Patients should continue to take their medications as directed by their healthcare provider. The ability of reSET-O to prevent potential relapse after therapy discontinuation has not been studied.
Clinicians should not use reSET-O to communicate with their patients about emergency medical issues. Patients should be clearly instructed not to use reSET-O to communicate to their clinician any urgent or emergent information. In case of an emergency, patients should dial 911 or go to the nearest emergency room.
This Press Release does not include all the information needed to use reSET-O safely and effectively. Please see the Clinician Brief Summary Instructions for reSET-O for more information.