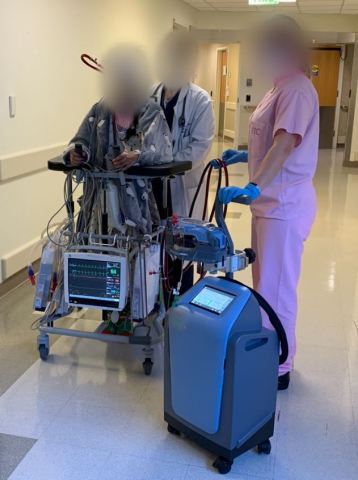
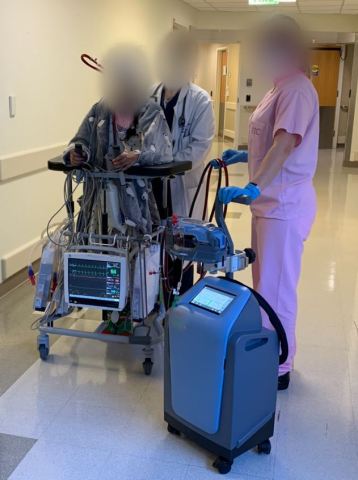
DANVERS, Mass.--(BUSINESS WIRE)--Abiomed (NASDAQ: ABMD) announces the first two patients in the world have been treated with the Abiomed Breethe OXY-1 System, a compact cardiopulmonary bypass system. The advanced ECMO technology pumps, oxygenates, and removes carbon dioxide from blood for patients whose lungs can no longer provide sufficient end organ oxygenation.
The system can help provide oxygenation to patients suffering from cardiogenic shock or respiratory failure from ARDS, H1N1, SARS, or COVID-19. When used with the Impella heart pump, it can unload the heart and oxygenate the body, a combination therapy known as ECpella.
The first patient is a 21-year-old woman at the University of Maryland Medical Center who sustained a lung injury and was placed on V-V ECMO with the Breethe system. She immediately began to improve and was able to ambulate daily, as seen in this short video.
After eight days, the patient was successfully weaned off Breethe support and is no longer in the ICU. The patient was treated by Bartley Griffith, MD, the Hales Distinguished Professor of Surgery at University of Maryland, School of Medicine and Daniel Herr, MD, chief surgical critical care services and medical director cardiac surgery ICU at University of Maryland Medical Center.
The second patient is a 51-year-old woman being treated at HUMC/Hackensack Meridian Health for respiratory failure due to COVID-19. After 24 hours of V-V ECMO support with the Breethe system, she is stable and improving. The patient was treated by Mark Anderson, MD, chief of the division of cardiac surgery and cardiothoracic surgeon at the Heart and Vascular Hospital at HUMC/Hackensack Meridian Health.
“Breethe is an important new option for patients with COVID-19 who require ECMO therapy. It is simple and intuitive to use, highly portable and a very promising therapy with the potential to help many patients,” said Dr. Anderson.
“This revolutionary technology will change the way we think about extracorporeal life support therapy by enabling physicians to easily mobilize their patients in turn promoting a faster recovery,” said Dr. Griffith. “I am pleased to be a part of this milestone as Abiomed continues to advance respiratory and cardiac support to improve patient outcomes.”
“The Breethe system significantly facilitates early mobilization and physical rehabilitation, which is critically important in the recovery of our patients,” said Chris L. Wells, PhD, PT, CCS, ATC, associate professor at the University of Maryland, School of Medicine, EBP, and research coordinator in the department of rehab services at University of Maryland Medical Center. She is also member of the medical team who treated the first patient.
Breethe adds to Abiomed’s innovative portfolio focused on native heart and lung recovery. Abiomed now provides the treatment options of the catheter-based Impella heart pump, which unloads the left ventricle, perfuses end organs and allows the heart to rest and recover, Breethe, which provides oxygenation, and the combination therapy of ECpella, when Impella and Breethe work together to unload the heart and oxygenate the body.
The Breethe system received FDA 510(k) clearance in October. It is being rolled out through a controlled launch at select hospitals in the United States, with full U.S. commercial availability expected in calendar year 2021.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support. Our products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart. For additional information, please visit: www.abiomed.com.
ABOUT ABIOMED BREETHE OXY-1 SYSTEM
The Abiomed Breethe OXY-1 System™ is cleared by the U.S. Food and Drug Administration (FDA) to provide extracorporeal circulation. The OXY-1 System pumps, oxygenates and removes carbon dioxide from blood during cardiopulmonary bypass up to six (6) hours in duration.
Under guidance issued by FDA, on April 6, 2020, the Abiomed Breethe OXY-1 System is now permitted to be used temporarily in the U.S. for ECMO therapy greater than six hours. Therefore, it now has a limited indication modification for use longer than six hours in an extracorporeal membrane oxygenation (ECMO) circuit to treat patients who are experiencing acute temporary respiratory or acute cardiopulmonary failure. This limited indication modification for ECMO therapy greater than six hours has not been cleared or approved by FDA and is in effect only for the duration of the public health emergency related to COVID-19 as declared by the U.S. Department of Health and Human Services (HHS).
FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements. Forward-looking statements are subject to risks and uncertainties such as those described in Abiomed's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.