SALT LAKE CITY--(BUSINESS WIRE)--Molecular diagnostics company XCR Diagnostics has been awarded a patent by the United States Patent and Trademark Office for its technology that speeds up the testing and identification process for infectious diseases. This reduces time-to-diagnosis from several hours to as little as 20 minutes with existing testing platforms, empowering even small public health laboratories to obtain rapid, actionable results. An initial key application will be for testing of the 2019-novel coronavirus, or COVID-19.
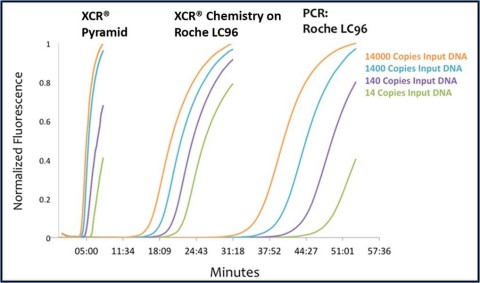
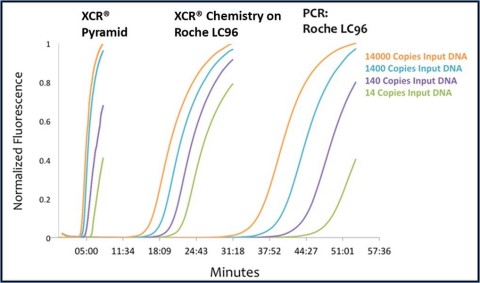
The new patent covers conversion of existing Polymerase Chain Reaction (PCR) assays to the company’s rapid chemistry format, Xtreme Chain Reaction (XCR®). While PCR technology is extremely effective in identifying and measuring infectious diseases and for forensic testing, it can take from one to several hours to complete a single test on a PCR-based testing platform. XCR supercharges the process on existing testing devices, more than doubling throughput and decreasing the wait time for results.
“Our unique intellectual property differentiates XCR from other nucleic acid amplification technologies by using existing conserved regions of the genome to identify infectious diseases in just 20 minutes on existing PCR instruments,” said Dr. Brian Caplin, chief scientific officer of XCR Diagnostics. “The ability to use our technology on any PCR system was key to our goal of impacting human health. We can now deploy XCR across a huge installed base of systems, allowing even small labs to do significant testing.”
“With the sudden global expansion of the novel coronavirus, it’s critical to make our technology available as soon as possible to help identify and sequester actual COVID-19 patients and carriers from the huge backlog of potential exposures,” stated Mark Powelson, chief executive officer of XCR Diagnostics. “We’re confident that we can have a COVID-19 test ready within weeks for deployment through the FDA emergency use authorization (EUA) or for consideration by health authorities in other countries.”
The Pyramid Portable Nucleic Acid Testing System
XCR Diagnostics is also developing a proprietary portable instrument system called the Pyramid that will take advantage of patented XCR technology to perform on-demand testing in ways conventional PCR systems cannot. The Pyramid is smaller, lighter, and faster than PCR systems, providing actionable results in less than 10 minutes. Because of its compact size and flexible power requirements, it can be easily and cost-effectively deployed at point of care in the field, making it invaluable during a disease outbreak in hard-to-reach or medically underserved areas. Three tests are currently under development for the Pyramid with FDA clinical trials planned for this year.
Visit XCR Diagnostics in booth 525 at the 2020 ASM Clinical Virology Symposium to be held May 3-6 in West Palm Beach, Florida.
About XCR Diagnostics
Founded in 2015, XCR Diagnostics Inc. is focused on delivering hyper-accurate molecular diagnostics testing near point of care to reduce healthcare costs and improve time-to-results for patients and central laboratories. The company is providing near-patient portable molecular diagnostics instrumentation, sample delivery systems, amplification, detection and analysis worldwide. Its patented technology converts existing PCR assays to the company’s rapid chemistry format, Xtreme Chain Reaction (XCR®). The Pyramid, its proprietary small portable nucleic acid testing system, combines with revolutionary molecular reagents to trim test times dramatically, positioning XCR to provide the first sub-10-minute molecular diagnostics test. Visit www.xcrdiagnostics.com for more information on partnering with XCR Diagnostics or licensing its technology.