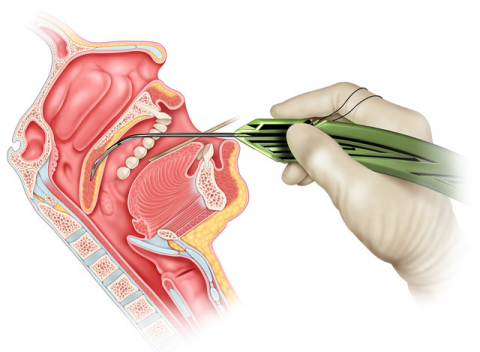
BLOOMINGTON, Ind.--(BUSINESS WIRE)--Today, Cook Medical and Zelegent, Inc., announced an FDA clearance for the Elevo® Kit Snoring Intervention Device. The specialized tool is designed to enhance patient treatment options for snoring. Elevo’s delivery system allows placement of specially-shaped sutures that provide lift to the soft palate and then are naturally dissolved by the body. It is indicated for symptomatic, habitual, and social snoring due to palatal flutter.
Elevo is used in Elevoplasty®, a procedure that provides lift and stiffening to the soft palate tissue in the mouth. Elevoplasty can be performed under local anesthesia in an office setting without the need for surgery.
“Elevoplasty is like a mini-face lift for the soft palate,” said Alexander K. Arrow, MD, chief executive officer of Zelegent. “We are honored to have the Elevo product adopted into a minimally invasive and innovative Cook product portfolio whose reputation dates back to Cook’s early work supporting pioneering physicians.”
Zelegent recently completed a multi-center clinical trial designed to evaluate the safety and efficacy of snoring intervention with Elevoplasty in an office-based setting. The S.I.L.E.N.C.E. clinical study included both academic institutions and private practitioners, including several thought leaders of sleep disorder treatments and international lecturers on sleep surgery in the field of otolaryngology.
“Approximately 20 percent of adults in the developed world consistently snore at night, at volumes high enough to disturb their sleeping partners,” said Thomas Cherry, OHNS global business leader. “This can be damaging to one’s quality of sleep, as well as their relationships. We’re very excited to provide a minimally invasive, office-based procedure to treat patients with this condition.”
Cook will begin training physicians on the Elevo Kit later this year.
About Zelegent, Inc.
Zelegent, Inc. is a medical technology company founded and majority-owned by sleep disorder specialist physicians to create innovative tools to treat sleep disorders. The company developed and grew in EvoNexus, Southern California’s leading corporate incubator. The company’s mission is to develop and provide minimally invasive devices to effectively alleviate primary snoring. Elevoplasty™ is a patented, office-based procedure. For updates, find Zelegent online at zelegent.com, or on Facebook, Twitter, LinkedIn, and Pinterest.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today, we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: patients, our employees, and our communities. Find out more at cookmedical.com, and for the latest news, follow us on Twitter, Facebook, and LinkedIn.