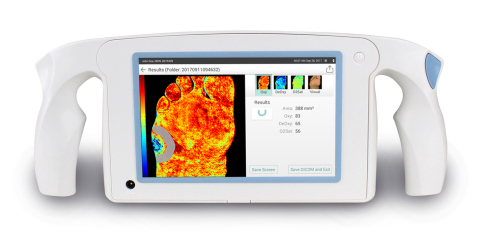
MEMPHIS, Tenn.--(BUSINESS WIRE)--HyperMed Imaging, Inc. announces that it has received approval to apply the CE Mark for the HyperView™ medical imaging system which will allow distribution in Europe. HyperView is also currently distributed in the United States.
The HyperView System is a fast, handheld, battery operated, non-invasive and portable diagnostic imaging device that is used to assess tissue oxygenation without contacting the patient. The system uses proprietary technology to capture a diagnostic image containing data for oxyhemoglobin levels (Oxy), deoxyhemoglobin levels (Deoxy) and oxygen saturation (O2Sat) in superficial tissue. Published literature has demonstrated that HyperView may assist the clinician in determining if a wound has adequate blood perfusion to heal. Likewise the clinician can assess if a vascular intervention or additional therapy is needed to improve perfusion.
Mark Darty, HyperMed’s President and CEO stated, “Receiving CE Mark for our HyperView product is a major milestone in our global commercialization efforts and we look forward to launching sales in the European market this year. HyperView is a leap forward in non-invasive assessment of vascular perfusion and tissue oximetry. HyperMed will have a marketing presence at vascular, wound care and reconstructive surgery conferences in Europe this year. We look forward to engaging key opinion leader medical professionals throughout Europe for the introduction of our class leading product and technology.”
Dr. Bauer Sumpio, Yale Medical School Professor of Surgery, Radiology and Medicine commented, “The HyperView product provides significant benefit in determining regional foot perfusion adequacy in vascular compromised patients. It is fast, easy and provides reliable diagnostics in comparison to existing standard of care devices. Endovascular surgery, wound care, and reconstructive surgery are all important areas which could benefit from use of the product. Its use as a screening tool prior to vascular interventions or various wound healing therapies can document the need for the procedure and its use post procedure can document the success and effectiveness of such procedures.”
HyperMed Imaging, Inc. is a privately owned medical device company located in Memphis, TN focused on vascular imaging. HyperMed pioneered the use of visible hyperspectral imaging for assessment of tissue oximetry in the superficial capillary bed. HyperMed’s unique use of visible light hyperspectral technology as opposed to use of near infrared light is to avoid mixing data from subcutaneous arteries and veins with that from the superficial capillary bed where oximetry data is different. Therefore, as demonstrated by published clinical research, HyperMed’s technology allows for consistent readings on which perfusion adequacy levels can be assessed. HyperMed’s core technology has over 12 years of established clinical efficacy and many journal publications. With over 60 issued patents and more than 40 additional patents pending worldwide, HyperMed is the market leader in advanced hyperspectral medical imaging. We invite key opinion leader physicians, customers and potential commercial partners worldwide to contact us for more information.
For more information, visit: https://www.hypermed.com/
HyperMed and HyperView are trademarks of HyperMed Imaging, Inc.
101-D114 Rev. A