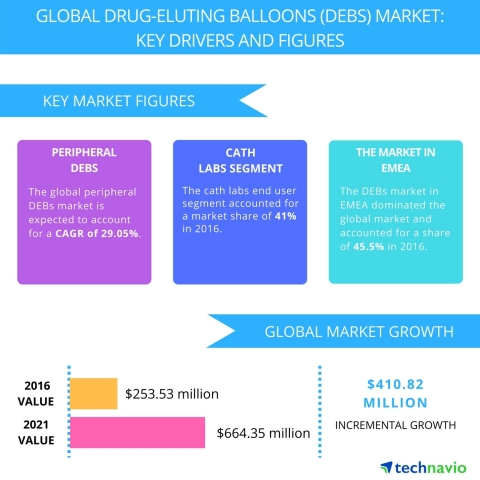
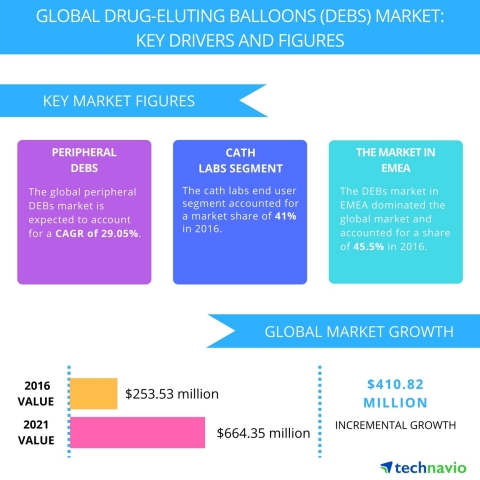
LONDON--(BUSINESS WIRE)--Technavio analysts forecast the global drug-eluting balloons market to grow to USD 664.35 million by 2021, at a CAGR of more than 27% over the forecast period, according to their latest report.
The research study by Technavio on the global drug-eluting balloons (DEBs) market for 2017-2021 provides a detailed industry analysis based on the product (peripheral DEBs and Coronary DEBs), end-user (cath labs, hospitals, and ambulatory surgical centers), and geography (EMEA, the Americas, and APAC).
EMEA currently leads the global DEB market due to the rise in peripheral arterial surgeries. However, the Americas is expected to establish market dominance by the end of the forecast period, the rise in the number of patients suffering from coronary and peripheral arterial diseases. Also, the introduction of new techniques and technological advances will boost the revenue generation.
This report is available at a USD 1,000 discount for a limited time only: View market snapshot before purchasing
Buy 1 Technavio report and get the second for 50% off. Buy 2 Technavio reports and get the third for free.
Technavio analysts highlight the following three factors that are contributing to the growth of the global drug-eluting balloons market:
- Growing demand for cath labs
- High growth potential of the US market
- Shift towards minimally invasive techniques
Growing demand for cath labs
“Cath labs in hospitals conduct both diagnostic and therapeutic procedures. As a part of treatment for coronary heart diseases, physicians perform coronary angiography and PCI for diagnosis. These procedures help confirm the severity of the disease, allowing physicians to determine treatment plans,” says Barath Palada, a lead analyst at Technavio for cardiovascular devices research.
Advances in technology in the area of catheterization, such as robot-assisted catheterization for percutaneous coronary intervention (PCI), have enabled advanced digital imaging to improve the detection and intervention related to cardiac diseases. This has encouraged the rise in the number of cath labs, and thereby the demand for DEBs. New cath labs are being established in hospitals and cardiac centers in tier II and tier III cities of emerging economies such as India, Brazil, and China.
High growth potential of the US market
DEBs were not available in the US market until 2014 owing to pending FDA approvals. Post the US FDA DEB approval in 2014, the market has been growing phenomenally and is expected to maintain its pace in the coming years. The US FDA first approved Lutonix 035 for the treatment of popliteal arteries, and then for IN.PACT Admiral from Medtronic for the treatment of de novo lesions.
Ongoing clinical trials and tentative approvals during the forecast period are expected to boost the demand for DEBs further. For instance, companies such as Eurocor, B. Braun Melsungen, and Cook Medical, which have significant shares in the European market are seeking the US FDA approval for their DEBs.
Shift towards minimally invasive techniques
Open heart surgeries and open peripheral surgeries involve many complications and can sometimes be life-threatening. Surgeons show a clear preference towards minimally invasive (MI) techniques as they are highly accurate, make for faster recovery times, are less painful, cause fewer post-surgery infections, reduce the hospital stays, provides more control on bleeding, and has minimal complications.
“The number of MI procedures to treat cardiac disorders has increased due to their advantages, thereby driving the demand for drug-eluting balloons. MI techniques help to enhance surgical precision with a 3D view of the patient, allowing manipulation of small surgical tools for the insertion of drug-eluting balloons in an individual's body,” says Barath.
Browse Related Reports:
- Global Mechanical Thrombectomy Devices Market 2017-2021
- Global Coronary Atherectomy Devices Market 2017-2021
- Global Cardiac Ablation Market 2017-2021
Become a Technavio Insights member and access all three of these reports for a fraction of their original cost. As a Technavio Insights member, you will have immediate access to new reports as they’re published in addition to all 6,000+ existing reports covering segments like orthopedics and medical devices, cardiovascular and metabolic disorders, and in-vitro diagnostics. This subscription nets you thousands in savings, while staying connected to Technavio’s constant transforming research library, helping you make informed business decisions more efficiently.
About Technavio
Technavio is a leading global technology research and advisory company. The company develops over 2000 pieces of research every year, covering more than 500 technologies across 80 countries. Technavio has about 300 analysts globally who specialize in customized consulting and business research assignments across the latest leading edge technologies.
Technavio analysts employ primary as well as secondary research techniques to ascertain the size and vendor landscape in a range of markets. Analysts obtain information using a combination of bottom-up and top-down approaches, besides using in-house market modeling tools and proprietary databases. They corroborate this data with the data obtained from various market participants and stakeholders across the value chain, including vendors, service providers, distributors, resellers, and end-users.
If you are interested in more information, please contact our media team at media@technavio.com.