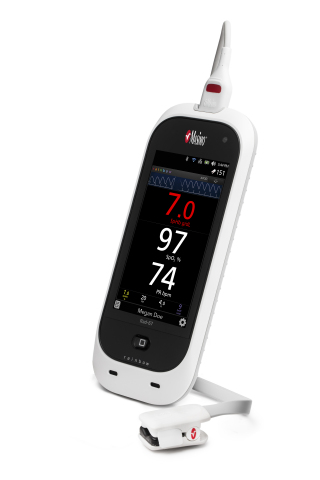
NEUCHATEL, Switzerland--(BUSINESS WIRE)--Masimo (NASDAQ:MASI) announced today, in conjunction with its CE marking, the limited market release of the Spot-Check Rad-67™ Handheld Pulse CO-Oximeter®. Rad-67 offers Measure-through Motion and Low Perfusion™ SET® pulse oximetry and upgradeable rainbow® noninvasive monitoring technology in a compact, portable spot-check device. With the universal reusable rainbow® DCI®-mini sensor, Rad-67 features Next Generation SpHb® (noninvasive total hemoglobin) technology.
Next Generation SpHb technology offers improved motion tolerance and a faster time to display SpHb results (in as few as 30 seconds). In addition, field performance has been enhanced in lower hemoglobin ranges. The rainbow® DCI-mini sensor allows patients of all ages, from infants to adults, to be spot-checked using a reuseable sensor. Next Generation SpHb, enabled when Rad-67 and the DCI-mini sensor are used together, significantly advances the forefront of noninvasive portable hemoglobin spot-checking.
Rad-67’s ability to provide portable spot-check measurements of both oxygen saturation and SpHb, in addition to other noninvasive rainbow® measurements, makes it a useful single-device solution in multiple care areas and mobile environments, such as when screening patients during blood donation drives, emergency room screening, and in physicians’ offices. In addition to a rechargeable battery with six-hour run time, Rad-67 features a high-resolution, HD color display with intuitive touchscreen navigation that automatically adjusts brightness to optimize visibility in a variety of settings, including outdoors. The slim-profile sensor connector port is designed to provide tactile feedback upon proper connection. Multiple parameters are simultaneously displayed, allowing for quick patient assessment.
Rad-67 provides convenient historical data review directly on the device, with unique patient identifiers to help improve organization of records and workflow. Using Bluetooth®, Rad-67 can connect wirelessly to portable printers. Rad-67 also includes built-in wireless connectivity via Wi-Fi. When used in conjunction with Masimo Iris Gateway™ or Patient SafetyNet™*, data from Rad-67 can be sent directly to the patient’s electronic medical record (EMR). Automating data transfer with EMR integration may help reduce the possibility of human error when manually transferring data.
Dr. Aryeh Shander, Chief of Anesthesiology, Critical Care Medicine at Englewood Hospital and Medical Center, noted that “in the process of screening for anemia, having the ability to detect hemoglobin noninvasively is one of the greatest advances. Once low hemoglobin is reported, confirmation with a conventional laboratory test will further explore the possible causes.”
Joe Kiani, Founder and CEO of Masimo, said, “We’re proud to announce Rad-67 with Next Generation SpHb technology. Next Generation SpHb represents a significant enhancement to the noninvasive measurement we invented a decade ago – a measurement we look forward to continuing to improve.”
Rad-67 has not received 510(k) clearance and is not available for sale in the United States.
@MasimoInnovates | #Masimo
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 including 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (PI). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/cpub/clinical-evidence.htm.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
**Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
|
References |
||||
|
1. |
Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92. |
|||
|
2. |
de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338. |
|||
|
3. |
Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287. |
|||
|
4. |
Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012. |
|||
|
5. |
McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302. |
|||
|
6. |
Estimate: Masimo data on file. |
|||
|
7. |
http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview. |
|||
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Rad-67™ Pulse CO-Oximeter®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-67, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.