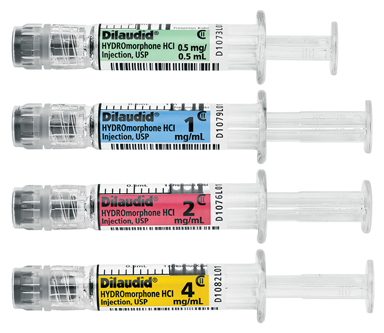
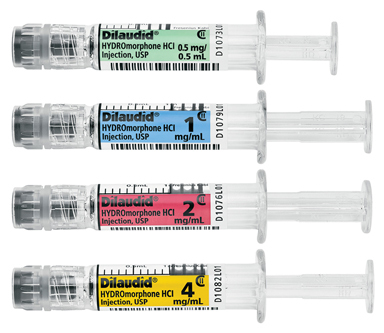
LAKE ZURICH, Ill.--(BUSINESS WIRE)--Fresenius Kabi announced today the immediate availability in the United States of Dilaudid® (HYDROmorphone HCl) Injection, USP in four presentations of Simplist™ ready-to-administer prefilled syringes.
Fresenius Kabi is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition.
Fresenius Kabi now offers Dilaudid in the following commonly used strengths:
- 0.5 mg per 0.5 mL
- 1 mg per 1 mL
- 2 mg per 1 mL
- 4 mg per 1 mL.
“Medication errors due to ‘look-alike’ and ‘sound-alike’ names are real, well documented and can have tragic outcomes for patients,” said John Ducker, president and chief executive officer of Fresenius Kabi USA. “Fresenius Kabi Simplist Dilaudid prefilled syringes can help prevent name confusion between hydromorphone and morphine and to assist health care professionals in delivering the right medication to their patients.”
The Institute for Safe Medication Practices (www.ismp.org) includes morphine and hydromorphone on its “List of Confused Drug Names.”
All Simplist ready-to-administer prefilled syringes, including the new Dilaudid presentations, are designed to offer clear and consistent labeling and feature individual bar codes on both packaging and syringes. The syringes are ready-to-administer meaning they require no assembly or point-of-care preparation, and they have a 24-month shelf life.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany.