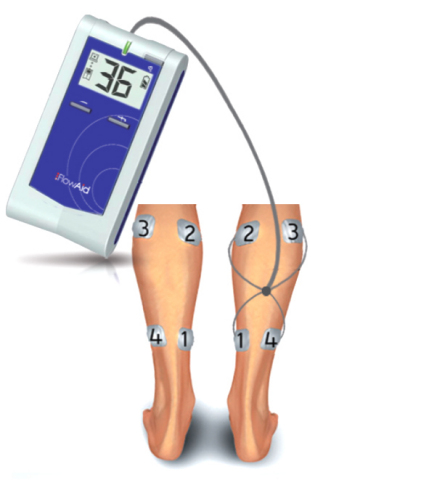
NEW YORK--(BUSINESS WIRE)--FlowAid Medical Technologies Corp., a U.S.-based company, has received CE mark approval for its FA100 SCCD Sequential Contraction Compression Device.
The FA100 SCCD is a breakthrough in the delivery of sequential compression therapy. After many years of R&D and the involvement of many multidisciplinary professionals, FlowAid has perfected a new approach enabled by patient feedback in the administration of sequential compression therapy. The FA100 SCCD was designed with the patient in mind – it is easy to use, allows complete mobility and operates without external pressure to the leg.
Sequential compression is the current standard of care for treating many forms of circulatory disorders in the lower extremities. Until now, this was achieved with external pressure to the leg. Now FlowAid can provide care for treating many forms of circulatory disorders in the lower extremities through a number of electrically stimulated controlled contractions.
The primary mechanism of the FlowAid FA100 SCCD is to compress the veins in the lower leg by using specifically controlled wave of peristaltic contractions, distal to proximal, causing an increase in Venous Outflow from the limb. This is useful in the treatment of Chronic Venous Insufficiency, Lymphedema, and the complications of these diseases. Keeping a constant flow will also prevent Deep Vein Thrombosis (DVT) from occurring. Secondarily, by increasing Venous Outflow, the FA100 SCCD also increases Arterial Inflow. This is useful for the treatment of Peripheral Arterial Disease (PAD), Arterial Insufficiency and the complications that may develop from these conditions. The FA100 SCCD has been useful in treating Microischemic Neuropathy by hyperperfusing the limb.
At FlowAid Medical Technologies Corp. our mission is to improve the quality of patient care through the development of non-invasive medical devices for increasing blood flow and circulatory enhancement. This is accomplished through the continuing refinement of existing products and the development of new vascular health care delivery technologies that can reduce risk, trauma, cost, procedure time and the need for aftercare.