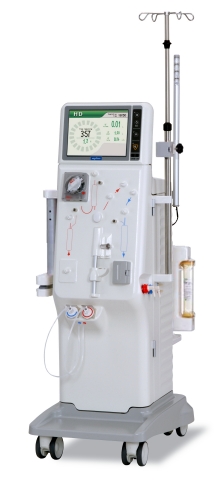
SAN DIEGO--(BUSINESS WIRE)--The Medical Division of Nikkiso America, Inc., announced today the U.S. launch of the DBB-06 Hemodialysis System equipped with Dialysis Dose Monitor (DDM), after receiving 510(k) clearance from the U.S. Food and Drug Administration (FDA) in March. The DBB-06 System with DDM was tailored to address U.S. dialysis clinic needs and it offers exceptional efficacy, efficiency and reliability at an affordable life cycle cost. This launch marks the entrance into the U.S. renal market for Nikkiso Medical.
Nikkiso Medical is one of the world’s leading manufacturers of dialysis machines and a market leader in Europe and Asia. The company is the second largest producer of hemodialysis equipment globally with over 100,000 hemodialysis machines in clinical use.
“After achieving a strong position in the global markets due to our outstanding product reliability, we are now providing a new option in hemodialysis equipment for U.S. clinics,” said Thomas Kelly, president of Nikkiso America’s Medical Division. “Nikkiso America is committed to continuing our company’s legacy of innovation to improve hemodialysis treatment outcomes while also addressing the needs of the care team.”
A unique feature integrated into the DBB-06 System is the Blood Volume Monitor, designed to help the clinician evaluate and react to the patient’s hydration status by continuously monitoring relative blood volume during treatment. The Blood Volume Monitor does not require additional disposables or cost per treatment. The DBB-06 System with DDM also includes:
- A full range of features, such as ultrafiltration profiling, ultrapure dialysate, patient blood pressure monitoring
- A self-prompting, intuitive touchscreen user interface and the storage and recall of up to 16 patient prescriptions
- Enhanced automation, with programmed self-test/extracorporeal circuit priming and automatic disinfection and decalcification, saving time
- Data connection to the electronic medical record (EMR)
- Exceptional quality and reliability
- The Dialysis Dose Monitor to assist the care provider in real-time to ensure an adequate treatment for the patient
- Safety features, including a battery backup system in the case of power failure and continuous monitoring of the fluid removal system
- Extremely quiet operation
The DBB-06 Hemodialysis System with DDM is now available across the country. To learn more about the DBB-06 Hemodialysis System equipped with Dialysis Dose Monitor, visit: http://nikkisoamerica.com/dbb-06-dialysis-system/.
About Nikkiso America
Nikkiso America, Inc. is headquartered in San Diego, Calif., and has offices and divisions based around the U.S., operating Nikkiso Co., Ltd.’s U.S.-based Medical, Aerospace and Industrial Divisions. The businesses include acute blood purification products, chronic dialysis systems, carbon fiber products for aerospace applications, pump products and fluid technologies, water conditioning systems for thermal and nuclear power plants, and particle size characterization. To learn more, visit www.nikkisoamerica.com.
Based in Tokyo, Japan, Nikkiso Co., LTD. (TYO: 6376), is recognized around the world for its 50-year history developing original innovative technologies that span many fields. The company manufacturers exquisitely engineered products from Nikkiso’s Medical, Aerospace, and Industrial Divisions and is the second largest producer of Hemodialysis Systems. To learn more, visit www.nikkiso.com.