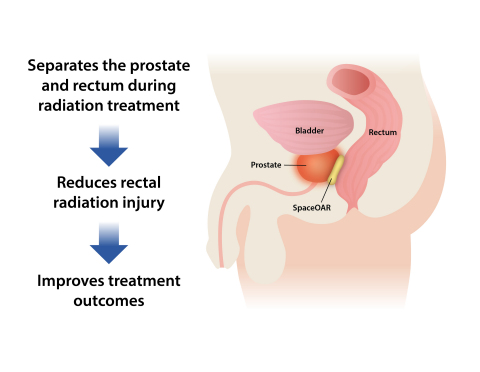
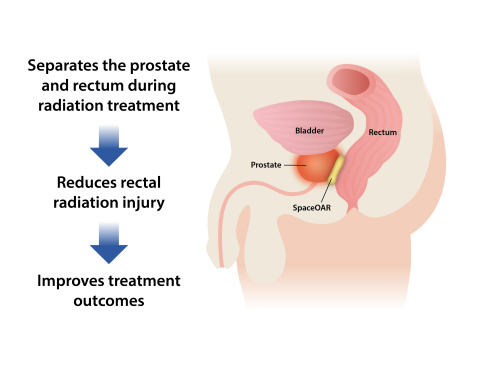
WALTHAM, Mass.--(BUSINESS WIRE)--Augmenix, Inc., a privately held company developing minimally invasive hydrogel products to improve outcomes following cancer radiotherapy, today announced the publication of the SpaceOAR® System US Clinical Trial results of the application technique, and impact on prostate intensity modulated radiation therapy (IMRT). Designed to act as a temporary spacer, the absorbable hydrogel pushes the rectum away from the prostate and reduces rectal radiation injury in men undergoing prostate cancer radiotherapy.
Published in the March issue of Urology Practice, an official journal of the American Urological Association (AUA) that focuses on clinical trends, challenges and practice applications, the paper describes outcomes in a randomized, controlled Level 1 clinical trial evaluating IMRT in prostate cancer patients with (SpaceOAR) and without (Control) the prostate-rectum spacing hydrogel.
“The simple concept of an absorbable spacer improving prostate radiotherapy outcomes makes so much sense,” said Lawrence I Karsh, MD, Director of Research and Attending Urologist, The Urology Center of Colorado and a study investigator. “I insist on the best care for my patients, and knowing this tool is now available increases my confidence in achieving excellent radiotherapy outcomes.”
Between 2010 and 2013, 222 patients with stage T1 or T2 prostate cancer were enrolled in 20 centers across the US. Either local, monitored or general anesthesia was used in an outpatient procedure to implant the hydrogel spacer. Patient tolerance of the spacer implantation procedure was deemed excellent, with only 10% of the SpaceOAR patients experiencing mild transient effects (e.g. transient perineal discomfort). The hydrogel spacer increased the prostate-rectum space by an average of 11mm (1.6 to 12.6mm), resulting in a significantly reduced radiation dose to the rectum with no signs of altering the prostate dose or increasing dose elsewhere. Median rectal (v70) radiation dose was decreased by 78% in SpaceOAR patients relative to control (p<0.0001).
As anticipated, the reduced rectal radiation improved outcomes. Patients in the SpaceOAR group experienced 76% less acute rectal pain events, a 71% reduction in the incidence of late rectal toxicity, and significant reductions in late rectal toxicity severity. In addition, patients in the SpaceOAR group experienced lower rates of decline in bowel quality of life. The hydrogel spacer was absorbed when evaluated 12 months after implantation and there were no implant infections, rectal wall complications or other issues related to the implant.
“It’s rare you find a product concept with all the arrows pointing in the right direction” said Amar Sawhney, Ph.D., company founder and Chairman of the Board. “Safely reducing rectal injury during prostate cancer radiotherapy will enable radiation delivery in fewer hospital visits, improving patient convenience, increasing facility throughput and saving healthcare dollars.”
The device received clearance from the U.S. Food and Drug Administration (FDA) in April 2015 and since then, more than 100 cancer treatment centers in 29 states and Washington, DC have adopted it. Augmenix will showcase the SpaceOAR System at the 111th AUA Annual Meeting, taking place May 6 - 10 in San Diego, CA.
The Augmenix Products
Using a minimally invasive procedure,
SpaceOAR System is injected as a liquid into the space between the
prostate and rectum where it expands the space and then solidifies into
a soft hydrogel. The hydrogel remains stable for three months while
protecting the rectum during radiotherapy, and then liquefies and is
completely absorbed. See the Instructions for Use for a complete list of
warnings, precautions and risks. The SpaceOAR System is CE marked and
TGA approved. Augmenix also markets TraceIT® Hydrogel, the world’s first
absorbable hydrogel tissue marker with CT, MRI and ultrasound
visibility. TraceIT Hydrogel is FDA cleared and CE marked, and is
finding utility as a marker to improve radiation targeting of bladder
tumors.
About Augmenix, Inc.
Augmenix, Inc. is a privately held
company based in the Boston area focused on the development and
commercialization of radiation oncology products using its proprietary
hydrogel technology. The company was founded by Incept LLC in 2008 and
is funded by several leading venture capital groups. More information
about Augmenix can be found at http://www.Augmenix.com.