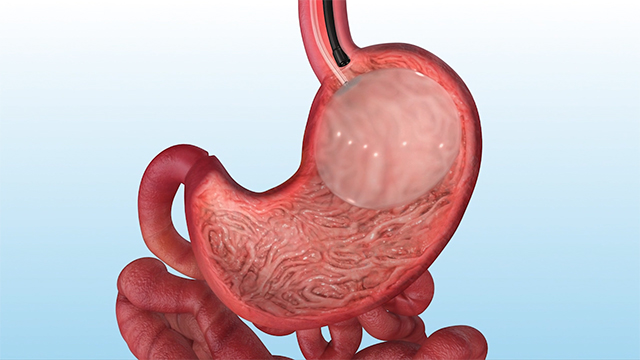
AUSTIN, Texas--(BUSINESS WIRE)--Apollo Endosurgery, Inc., a leader in minimally invasive endoscopic surgical products for bariatric and gastrointestinal procedures, today announced that the U.S. Food and Drug Administration (FDA) has approved the ORBERA™ Intragastric Balloon to assist adult patients suffering from obesity - with a body mass index (BMI) of 30 to 40 - in losing and maintaining weight.
The ORBERA™ balloon is part of the ORBERATM Managed Weight Loss System, a comprehensive, non-surgical two-part program that includes a balloon filling space in a patient’s stomach to reinforce proper portion control. Data on ORBERA™ collected in the U.S. clinical trial has shown that the average person lost 3.1 times the weight as compared with diet and exercise alone within six months.
“While new to the United States, ORBERA™ is a weight loss device with more than 220,000 balloons distributed in over 80 countries and approximately 230 published papers documenting its clinical results,” said Todd Newton, Chief Executive Officer of Apollo Endosurgery. “ORBERA™ is a proven, innovative and non-surgical solution to help fight the obesity epidemic and treat patients before their disease progresses and requires more invasive treatments. With the FDA approval of ORBERA™, Apollo can now offer this safe and effective solution to patients and their physicians in the United States.”
“For many, the weight loss journey leaves patients with little support or options other than diet and exercise and traditional surgery. The approval of ORBERA™ fills this gap in available treatments and is an exciting development for healthcare professionals who are committed to providing patients with less invasive options that can assist them in reaching their long-term weight loss goals,” said Christine Ren-Fielding, M.D., a New York-based bariatric surgeon. “ORBERA™ gives us a new weight loss option to help address what has become a critical health issue in the United States.”
Introducing ORBERATM
ORBERA™ is an incision-less, non-surgical weight loss solution designed for adult patients suffering from obesity, who are not appropriate for or considering invasive surgery, but for whom diet and exercise or pharmaceutical interventions have not worked.
In a non-surgical (endoscopic) procedure done under a mild sedative, the thin and deflated ORBERA™ balloon is placed into the stomach. It is then filled with saline until it’s about the size of a grapefruit. The procedure typically takes about 20 to 30 minutes and the patient can generally go home the same day. At six months, through another non-surgical procedure done under a mild sedative, the ORBERA™ balloon is deflated and then removed.
Once the balloon is in place, the patient receives an individually tailored support program through the ORBERATM Managed Weight Loss System team of experts - which may include a dietician, psychologist and exercise physiologist - to help keep them motivated, coordinate their program and help them work through weight loss barriers to meet their long-term weight loss goals. Coaching takes place over 12 months, even though the balloon is removed after six months. The program is designed to help the patient develop sustainable, healthy habits that will help keep weight off over time.
ORBERA™ Pivotal Study Results
In the U.S. pivotal ORBERA™ clinical trial, a multicenter, prospective, randomized, non-blinded comparative study, patients suffering from obesity with a BMI between 30 and 40 were randomized to treatment or control in a 1:1 ratio. The treatment group underwent placement of the ORBERA™ balloon followed by removal after six months. They concurrently participated in a 12-month behavioral modification program. The control group participated in the 12-month behavioral modification program alone. For patients in the treatment group, the device was removed at month six, with regular office visits continuing through one year.
A total of 125 patients were randomized to the treatment group and 130 patients were randomized to the control group.
Detailed findings from the trial include:
- At month six, the ORBERA™ group achieved a mean of 38.4 percent Excess Weight Loss (EWL).
- Mean Total Body Weight Loss (TBWL) at six months was 10.2 percent for the treatment group compared to 3.3 percent TBWL for the control group.
- The ORBERA™ group lost 3.1 times as much weight as the control group at six months.
- The ORBERA™ group also lost significantly more weight than the control group over the course of the study, and was able to maintain significant weight loss through month 12, six months after removal of the device.
For more than 20 years, the global medical community has been using intragastric balloons from the makers of ORBERA™ to help thousands of people lose weight. More than 220,000 ORBERATM balloons have been distributed worldwide in over 80 countries.
For additional information regarding ORBERA™, please visit Orbera.com.
For full safety information please visit orbera.com/dfu, talk with your doctor, or call Apollo Customer Support at 1-855-MYORBERA
About Apollo Endosurgery, Inc.
Apollo Endosurgery, Inc. is revolutionizing the treatment of obesity and other gastrointestinal disorders by developing less invasive solutions for a whole new group of patients not seeking treatment today. Apollo is a global innovator pushing boundaries to bring new technologies and innovative products to markets in over 80 countries today. In particular, Apollo’s bariatric products fill the gap between non-surgical weight loss solutions and invasive surgeries, allowing physicians to do more for those patients who require more than drug therapy and dietary advice. For more information regarding Apollo Endosurgery, go to: www.apolloendo.com.
© 2015 Apollo Endosurgery, Inc. All rights reserved. Any third-party trademarks used herein are the property of their respective owners.