BEDFORD, Mass.--(BUSINESS WIRE)--ConforMIS, Inc., a medical device company providing the only truly customized total knee implant systems for patients, today announced results from two in vivo clinical studies comparing the motion patterns of patients treated with ConforMIS’ iTotal® versus off-the-shelf knee implants. The studies involved the first-ever use of an advanced real-time mobile x-ray fluoroscopy system designed to measure a wider range of natural movements. Results were presented at the 2014 International Congress for Joint Reconstruction (ICJR) Pan Pacific Orthopaedic Congress.
Both studies demonstrated that the motion pattern and stability of customized ConforMIS implants, compared to traditional, off-the-shelf implants, more closely behaved like a normal knee. Replicating the motion of a patient’s knee is critical to achieving a more stable, natural feeling knee and achieving normal function. This is important since several independent studies have shown that approximately one in five patients are dissatisfied with traditional off-the-shelf knee replacements, with common causes being residual pain, unnatural feeling, and poor function. The ConforMIS total knee replacement is designed to eliminate causes of patient dissatisfaction common with traditional off-the-shelf implants, by matching not only the unique size but also the unique 3D shape of each patient’s knee.
“The goal of knee replacement surgery is for the patient to ultimately forget about the knee implant,” said Philipp Lang, MD, MBA, Chairman and CEO of ConforMIS. “The ability of the ConforMIS implant to more closely match the motion patterns of a normal knee is further confirmation that individually designed, customized implants are a true breakthrough in knee replacement technology.”
These studies follow a recently presented study at the British Association for Surgery of the Knee (BASK) Annual Meeting that showed 100% of patients in a series of 106 ConforMIS iTotal knee replacements reported a normal feeling knee.1 This new data adds to the growing body of evidence that a customized approach to implant design results in a knee that moves and feels more like a normal knee.
In the first study presented at the ICJR: “In Vivo Kinematics for Subjects Implanted With Either a Traditional or a Customized, Total Knee Replacement,” lead researcher William Kurtz, MD evaluated patients treated with the ConforMIS customized implant and compared them to patients treated with one of the leading traditional, off-the-shelf implants while they performed a variety of activities. Results of the study showed differences in the overall motion and pattern between the two subject groups:
- 91% of patients with a ConforMIS customized knee experienced a motion pattern and rotation consistent with the normal knee, compared to only 56% of patients with an off-the-shelf implant.
- 0% of ConforMIS patients had any abnormal lift off of the implant of either the inside (medial) or outside (lateral) portion of the knee, compared to 56% of traditional, off-the-shelf patients. Abnormal lift off of the implant is an indication of instability (a “wobbly” knee) and abnormal movement of the knee.
- Patients with a ConforMIS knee achieved greater overall range of motion compared to off-the-shelf patients.
“These findings show that ConforMIS patients experienced motion and stability that is consistent with a normal knee, while patients with an off-the-shelf knee implant experienced knee motion inconsistent with how a normal knee behaves,” said William Kurtz, MD, Saint Thomas Midtown Hospital in Nashville, TN.
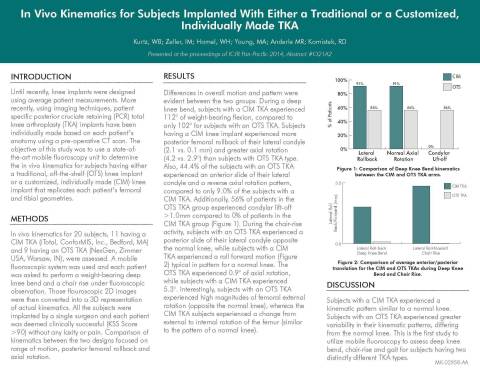
In the second study presented at the ICJR, “In Vivo Kinematics for Customized, Individually Made vs. Traditional Total Knee During a Deep Knee Bend and Chair Rise,” lead researcher Harold Cates, MD evaluated kinematics of patients with a ConforMIS customized implant versus another leading off-the-shelf implant while they performed basic movements. Patients were asked to perform a deep knee bend to measure an important function called femoral rollback to better understand if the knee replacement behaves like a normal knee. Results demonstrated that:
- All ConforMIS subjects experienced posterior femoral rollback of their lateral condyle, consistent with the motion of a normal knee, compared with only 50% of traditional, off-the-shelf subjects.
- Subjects in the ConforMIS arm achieved more natural rotation patterns and more normal contact patterns of the femur (thigh bone) and tibia (shine bone) compared to subjects with an off-the-shelf implant.
Finally, these studies are the first-ever results using an advanced real-time x-ray mobile fluoroscopic system developed by Dr. William Hamel and Dr. Richard Komistek to evaluate knee motion and kinematics. This mobile system allows researchers to monitor a wider range of natural movement. For example, rather than having patients walk on a treadmill, the mobile fluoroscopy unit follows patients as they walk normally.
About ConforMIS, Inc.
ConforMIS, Inc. is a privately-held medical device company that is pioneering a patient-specific approach to orthopedic implants and instrumentation. Its proprietary technology is supported by more than 375 patents and patent applications. ConforMIS’ award winning partial and total knee replacement solutions, the iUni® G2, iDuo® G2 and iTotal® G2, are individually designed for each patient. Potential advantages compared to traditional off-the-shelf implants include faster recovery, shorter hospital stay, less blood loss, more bone preservation, and better functional outcomes with more natural feeling knees. ConforMIS products are provided in a pre-sterilized single package delivery system that can help hospitals reduce costs and treat more patients by reducing instrument re-sterilization costs and shortening set-up, procedure and turnover times. The iUni® G2, iDuo® G2 and iTotal® G2, have been cleared by the U.S. FDA and are CE Marked in Europe.
ConforMIS is the leader in additive manufacturing in the orthopedic and medical device space. ConforMIS is the only orthopedic company that offers Just-In-Time (JIT) manufacturing with a single-package delivery system, resulting in greatly enhanced use of working capital.
ConforMIS technology is broadly applicable to all joints. The company is expanding into new applications and indications.
For more information visit www.conformis.com, follow @ConforMIS on Twitter, or ‘Like’ the ConforMIS Facebook page.
1 Kurtz, el al; Early Outcomes Utilizing a First-Generation Customized Patient-Specific TKA Implant, 2014 BASK Annual Meeting