CAMBRIDGE, Mass.--(BUSINESS WIRE)--PatientsLikeMe has unveiled a suite of services for pharmaceutical companies that allows them to collaborate with patients on the design of clinical trials and other research. The announcement was made at the 50th annual meeting of the Drug Information Association (DIA) in San Diego, where PatientsLikeMe was awarded the 2014 DIA President’s Award for Outstanding Achievement in World Health. The award, accepted on behalf of PatientsLikeMe members and staff by Co-Founder and Chairman Jamie Heywood, is the DIA’s highest, and recognizes significant and innovative contributions to the improvement of world health.
PatientsLikeMe Vice President of Innovation Paul Wicks, Ph.D. said that with more than 250,000 members reporting on their experiences with 2,000 diseases, PatientsLikeMe has transformed the way patients can shape research. “We’ve nurtured an open, data-sharing environment where members share their experiences to live better, and built a foundation for pharma-patient partnerships that can truly advance medicine. By listening to patients, we can create actionable insights that optimize the design of trial protocols and bring about better treatments, faster.”
The new services were launched as results from a March 2014 survey of PatientsLikeMe members reveal just how broken the trial process is, and how eager patients are to help fix it. Members were asked about their attitudes toward, experiences with and criteria for participating in clinical trials. Of 1,621 survey respondents:
- One out of three (31%) reported having previously been invited to learn about a clinical trial, and 62% of that group participated in one.
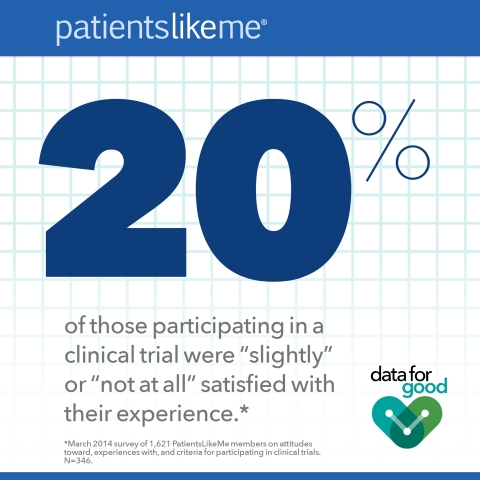
- Of those participating in a trial, one in five (20%) were “slightly” or “not at all” satisfied with their experience.
- An overwhelming majority (93%) said they would be willing to help researchers design better trials.
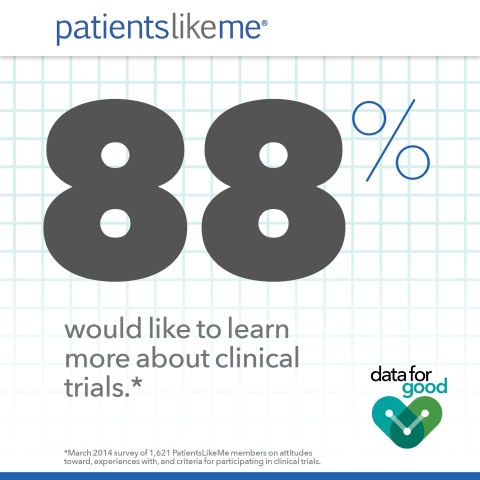
- Regarding future participation, 88% said they would like to learn more about clinical trials, while 80% indicated they would like to take part in a trial within the next 12 months.
The full survey results will be presented at DIA’s Professional Poster Session at location T44 on Tuesday, June 17, 2014.
PatientsLikeMe announced three services at DIA:
- Trial Access, which allows pharmaceutical companies to develop and deploy custom research programs that gather meaningful data and feedback on the design of their clinical trials. Trial Access also includes review of an expanding repository of patient opinions and attitudes about participating in clinical trials. Once the trial protocol is finalized, companies can run outreach and referral programs to raise awareness and recruit for their trial among PatientsLikeMe’s members, a highly effective service that PatientsLikeMe’s top pharmaceutical clients have used since 2008.
- Community Access, which pairs researchers with PatientsLikeMe scientists and patient engagement experts to build open, online registries for research to gain insights from patient-reported data that can be shared across the organization.
- Access Services, which allows companies to quickly collect and analyze real world data to generate statistically robust and scientifically credible patient outcome research, and determine the impact of new wearable and consumer-oriented health devices and sensors on clinical development and commercialization.
For more information, visit www.patientslikeme.com/services.
About PatientsLikeMe
PatientsLikeMe® (www.patientslikeme.com) is a patient network that improves lives and a real-time research platform that advances medicine. Through the network, patients connect with others who have the same disease or condition and track and share their own experiences. In the process, they generate data about the real-world nature of disease that help researchers, pharmaceutical companies, regulators, providers, and nonprofits develop more effective products, services and care. With more than 250,000 members, PatientsLikeMe is a trusted source for real-world disease information and a clinically robust resource that has published more than 50 peer-reviewed research studies. Visit us at www.patientslikeme.com or follow us via our blog, Twitter or Facebook.