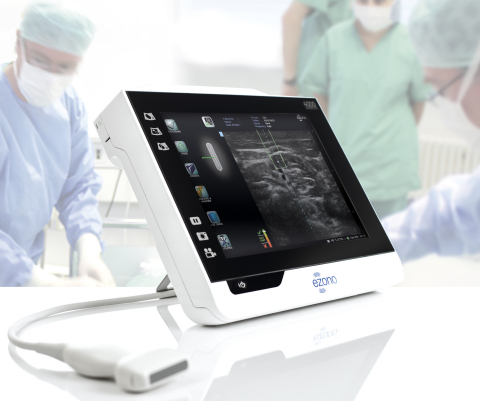
JENA, Germany--(BUSINESS WIRE)--eZono AG (“eZono”), a private corporation that designs, develops and markets state-of-the-art tablet ultrasound systems, today announced it has received CE marking in Europe for the eZono 4000 tablet ultrasound system, with eZGuide, a revolutionary needle guidance technology.
Due to the impact on patient safety and efficiency, procedural ultrasound guidance for Central Line Placement, Regional Anesthesia and Pain Management is a rapidly expanding market worldwide. The eZono 4000 is designed specifically for procedural ultrasound and features our proprietary eZGuide technology, the first freehand navigation system to visualize the position of the needle and location of the needle tip in real time, without the need for special needles or additional equipment.
“We are very excited to bring this advanced imaging and needle guidance technology to the market”, said Graham Cox, CEO of eZono., “Until now, identifying the needle, especially the needle tip when performing ultrasound guidance has been very challenging. The eZono 4000 delivers a combination of powerful features, enabling point of care clinicians to perform procedures with greater accuracy, increased confidence and having the ability to clearly identify the position of the needle tip”. Existing technologies are very limited and can add significant costs to each procedure, due to the requirement of specific additional equipment and/or special needles.
“When designing eZGuide, we studied at length the real life everyday problems faced by clinicians when performing point of care procedures, and consideration of the mounting costs endured by healthcare providers worldwide” explained Eliseo Sobrino, Chief Technology Officer. “We want to deliver a product that offers increased safety without changing established protocols whilst reducing costs. I believe we have achieved that goal with the eZono 4000”.
The eZono 4000 is currently available for sale in Europe and all countries accepting CE mark.
About eZono
eZono AG headquartered in Jena, Germany, designs, develops and distributes state of the art tablet ultrasound systems specifically targeted at point-of-care markets. The first generation eZono 3000 is CE marked and achieved FDA clearance in July 2011 and is now being successfully marketed and sold globally. The company is dedicated to supplying sophisticated imaging solutions to empower point-of-care providers.