MONMOUTH JUNCTION, N.J.--(BUSINESS WIRE)--Insmed Incorporated (Nasdaq GS:INSM) today reported results from the Company’s phase 2 clinical trial of ARIKAYCETM, or liposomal amikacin for inhalation, for the treatment of patients with treatment resistant nontuberculous mycobacterial (NTM) lung infections. The randomized, double-blind, placebo-controlled phase 2 clinical trial compared ARIKAYCE (590 mg delivered once daily), added to standard of care treatment, versus standard of care treatment plus placebo, in 90 adult patients with treatment resistant NTM lung disease. Eligibility for the study required patients to have been on the American Thoracic Society/Infectious Disease Society of America (ATS/IDSA) guideline therapy for at least six months prior to screening and to continue to have persistently positive mycobacterial cultures.
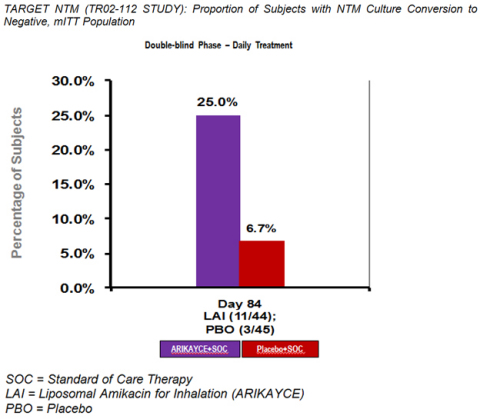
The primary efficacy endpoint of the study was a semi-quantitative measurement of the change in mycobacterial density on a seven-point scale from baseline (day one) to the end of the randomized portion of the trial (day 84). ARIKAYCE did not meet the pre-specified level for statistical significance although there was a positive trend (p=0.148) in favor of ARIKAYCE. However, ARIKAYCE did achieve statistical significance with regard to the clinically relevant key secondary endpoint of culture conversion, with 11 out of 44 patients treated with ARIKAYCE (added to standard of care treatment) demonstrating negative cultures by day 84 of the study as compared to 3 out of 45 patients treated with placebo (added to standard of care treatment) (p=0.01).
Patients receiving ARIKAYCE experienced a greater number of adverse events than those receiving placebo. All adverse events experienced with ARIKAYCE were consistent with those seen in similar patient populations receiving inhaled antibiotics. The most common side effect was laryngeal irritation.
The Company plans to incorporate these results into discussions with the regulatory agencies in the United States and Europe to determine next steps for ARIKAYCE. The Company also intends to apply for Breakthrough Therapy Designation for ARIKAYCE in the United States based upon the culture conversion results. ARIKAYCE has already received Orphan Drug, Qualified Infectious Disease Product (QIDP) and Fast Track designations from the U.S. Food and Drug Administration (FDA) for the treatment of NTM lung infections and recently received Orphan Drug Designation from the European Medicines Agency (EMA).
“The results relating to the key secondary endpoint of culture conversion are encouraging, and I believe demonstrate that ARIKAYCE has the potential to be an option for treating even the most difficult treatment resistant patients with NTM lung infections,” said David Griffith, M.D., Professor of Medicine, W.A and E. B. Moncrief Distinguished Professor at The University of Texas Health Sciences Center and a co-Principal Investigator on the study.
“We are encouraged by the achievement of culture conversion in this trial, which we believe is the ultimate goal in the treatment of mycobacterial infections,” said Dr. Renu Gupta, Executive Vice President, Development and Chief Medical Officer of Insmed. “The design of this trial was such that the patients who entered the trial and received drug were clearly resistant to guideline therapy, making them the most treatment-challenged NTM patients. Therefore the hurdle for showing any improvement with a therapy is extremely high.”
“While ARIKAYCE did not achieve statistical significance on the primary endpoint of bacterial density reduction, we are very pleased by the greater number of culture conversions among patients receiving ARIKAYCE,” stated Will Lewis, President and Chief Executive Officer of Insmed. “We now look forward to the regulatory discussions in the US and Europe that will guide our path forward.”
“We extend our gratitude to the investigators and their patients who participated in this study,” concluded Mr. Lewis.
Primary Endpoint
The objective of the primary endpoint was to show a reduction in the density of bacteria of at least one step along a seven point scale as a means of identifying whether a trend suggestive of ultimate culture conversion could be established. The number of patients showing at least a one-step reduction in the treatment arm versus those in the placebo arm was not statistically significant (p=0.148). The design of the study’s primary endpoint was not culture conversion because it was assumed that culture conversion would not be achievable in 84 days treatment, particularly given the severe, treatment-resistant patient population that was the subject of this study.
Secondary Endpoints
ARIKAYCE achieved statistical significance with regard to the secondary outcome of culture conversion, with 11 out of 44 patients treated with ARIKAYCE demonstrating negative cultures by day 84 of the study as compared to 3 out of 45 patients treated with placebo arm (standard of care therapy only) (p=0.01). Data analyses for additional secondary, tertiary and exploratory endpoints are ongoing and will be discussed at a presentation at the American Thoracic Society meeting in May.
Safety
Patients receiving ARIKAYCE experienced adverse events consistent with those seen in similar patient populations receiving inhaled antibiotics. Overall, mild to moderate throat irritation was more common in the ARIKAYCE arm compared to the placebo arm. One Suspected Unexpected Serious Adverse Reaction (SUSAR) was observed in the ARIKAYCE arm, but there was no difference in severe serious adverse reactions or hemoptysis between the two arms. Instances of hearing loss or tinnitus, a side effect more commonly associated with intravenous dosing of amikacin, were evenly balanced between the ARIKAYCE and placebo arms. The study population was chronically ill with a mean age of 61.
Clinical Trial Design
Mycobacterial density is a measurement currently used in clinical practice to assess the progress or decline of those patients with recalcitrant NTM. Following the randomized portion of the study, all eligible patients had the option to receive ARIKAYCE once daily for an additional 84 days in an open-label design.
Patients in the trial were stratified for either Mycobacterium avium complex (MAC) infections or Mycobacterium abscessus infections. These pathogens collectively account for approximately 85% of all patients with NTM lung disease in the U.S. Stratification was also performed based on patients with cystic fibrosis versus those who do not have cystic fibrosis.
Conference Call and Webcast
Insmed management will host an investment community conference call today at 8:00 a.m. Eastern time, featuring several clinical trial investigators who are experts in NTM. Shareholders and other interested parties may participate in the call by dialing 888-803-5993 (domestic) or 706-634-5454 (international) and referencing conference ID number 19390649. The call will be webcast live and archived at http://investor.insmed.com/events.cfm.
A replay of the conference call will be accessible two hours after its completion through April 1, 2014 by dialing 855-859-2056 (domestic) or 404-537-3406 (international) and referencing conference ID number 19390649. The call will also be archived for 90 days on the Company’s website at www.insmed.com.
About Nontuberculous Mycobacteria (NTM)
Nontuberculous mycobacteria (NTM) are organisms found in the soil and water that can cause serious lung disease in susceptible individuals, for which there are currently limited effective treatments and no approved therapies. The prevalence of NTM disease is reported to be increasing, and according to reports from the American Thoracic Society is believed to be greater than that of tuberculosis in the U.S. According to the National Center for Biotechnology Information, epidemiological studies show that presence of NTM infection is increasing in developing countries, perhaps because of the implementation of tap water. Women with characteristic phenotype are believed to be at higher risk of acquiring NTM infection along with patients with defects on cystic fibrosis transmembrane conductance regulators.
NTM lung disease is often a chronic condition that can lead to progressive inflammation and lung damage, and is characterized by bronchiectasis and cavitary disease. NTM infections often require lengthy hospital stays for medical management. Treatment usually involves multi-drug regimens that can be poorly tolerated and have limited effectiveness, especially in patients with severe disease or in those who have failed prior treatment attempts. According to a company-sponsored patient chart study conducted by Clarity Pharma Research, approximately 50,000 patients suffering from NTM lung disease visited physician offices in the U.S. during 2011.
About ARIKAYCE®
ARIKAYCE is a form of the antibiotic amikacin, which is enclosed in nanocapsules of lipid called liposomes. This advanced pulmonary liposome technology prolongs the release of amikacin in the lungs while minimizing systemic exposure. The treatment uses biocompatible lipids endogenous to the lung that are formulated into small (0.3 micron), charge-neutral liposomes. ARIKAYCE is administered once-daily using an optimized, investigational eFlow® Nebulizer System manufactured by PARI Pharma GmbH, a novel, highly efficient and portable aerosol delivery system.
This is the first controlled clinical trial of an antibiotic in patients suffering from NTM lung infections. There are no drugs approved by the FDA for the treatment of this chronic, debilitating disease.
About eFlow® Technology and PARI Pharma
ARIKAYCE is delivered by an investigational eFlow® Nebulizer System developed by PARI Pharma and optimized specifically for ARIKAYCE. The optimized device uses eFlow Technology to enable highly efficient aerosolization of medication including liposomal formulations via a vibrating, perforated membrane that includes thousands of laser-drilled holes. Compared with other nebulization technologies, eFlow Technology produces aerosols with a very high density of active drug, a precisely defined droplet size and a high proportion of respirable droplets delivered in the shortest possible period of time. eFlow Technology is not an ultrasonic nebulizer technology and is not a general purpose electronic aerosol generator nebulizer technology. Combined with its quiet mode of operation, small size, light weight and battery use, eFlow Technology reduces the burden of taking daily, inhaled treatments.
About Insmed
Insmed Incorporated is a biopharmaceutical company dedicated to improving the lives of patients battling serious lung diseases. Insmed is focused on the development and commercialization of ARIKAYCE™, or liposomal amikacin for inhalation, for at least two identified orphan patient populations: patients with nontuberculous mycobacteria (NTM) lung infections and cystic fibrosis (CF) patients with Pseudomonas aeruginosa lung infections. For more information, please visit http://www.insmed.com.
Forward-looking Statements
This release contains forward-looking statements. Words, and variations of words, such as “intend,” “expect,” “will,” “anticipate,” “believe,” “continue,” “propose” and similar expressions are intended to identify forward-looking statements. Investors are cautioned that such statements in this release, including statements relating to the status, results and timing of clinical trials and clinical data, the anticipated benefits of Insmed’s products, the anticipated timing of regulatory submissions, and the ability to obtain required regulatory approvals, the ability to obtain a Breakthrough Therapy Designation for ARIKAYCE in the U.S., bring products to market and successfully commercialize products constitute forward-looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, without limitation, failure or delay of European, Canadian, U.S. Food and Drug Administration and other regulatory reviews and approvals, competitive developments affecting the Company’s product candidates, delays in product development or clinical trials or other studies, patent disputes and other intellectual property developments relating to the Company’s product candidates, unexpected regulatory actions, delays or requests, the failure of clinical trials or other studies or results of clinical trials or other studies that do not meet expectations, the fact that subsequent analyses of clinical trial or study data may lead to different (including less favorable) interpretations of trial or study results or may identify important implications of a trial or study that are not reflected in Company’s prior disclosures, and the fact that trial or study results or subsequent analyses may be subject to differing interpretations by regulatory agencies, the inability to successfully develop the Company’s product candidates or receive necessary regulatory approvals, inability to make product candidates commercially successful, changes in anticipated expenses, changes in the Company’s financing requirements or ability raise additional capital, and other risks and challenges detailed in the Company’s filings with the U.S. Securities and Exchange Commission, including its Annual Report on Form 10-K for the year ended December 31, 2013. Investors are cautioned not to place undue reliance on any forward-looking statements that speak only as of the date of this news release. The Company undertakes no obligation to update these forward-looking statements to reflect events or circumstances or changes in its expectations.
Select ARIKAYCE™ Clinical Data
|
TARGET NTM (TR02-112 STUDY): Change from Baseline on the Full Semi Quantitative Scale for Mycobacterial Culture, mITT Population |
||||||
| Day 84 |
ARIKAYCE |
Placebo |
Overall |
|||
| -6 | 1 (2.3) | 0 | 1 (1.1) | |||
| -5 | 0 | 0 | 0 | |||
| -4 | 1 (2.3) | 0 | 1 (1.1) | |||
| -3 | 3 (6.8) | 1 (2.2) | 4 (4.5) | |||
| -2 | 4 (9.1) | 6 (13.3) | 10 (11.2) | |||
| -1 | 9 (20.5) | 6 (13.3) | 15 (16.9) | |||
| 0 | 20 (45.5) | 20 (44.4) | 40 (44.9) | |||
| +1 | 3 (6.8) | 7 (15.6) | 10 (11.2) | |||
| +2 | 0 | 3 (6.7) | 3 (3.4) | |||
| +3 | 1 (2.3) | 0 | 1 (1.1) | |||
| +4 | 1 (2.3) | 2 (4.4) | 3 (3.4) | |||
| +5 | 0 | 0 | 0 | |||
| +6 | 0 | 0 | 0 | |||
| +7 (Death) | 1* (2.3) | 0 | 1 (1.1) | |||
| * Death determined to be not related to study drug; data handling rules require any death to be scored as a +7 | ||||||
|
TARGET NTM (TR02-112 STUDY): Summary of semi quantitative data for mITT population |
|||||||||||
| ARIKAYCE | Placebo | ||||||||||
| # of Patients |
Cumulative |
# of Patients |
Cumulative |
||||||||
|
Patients with decrease in bacterial |
18 | -36 | 13 | -21 | |||||||
| Patients with no change on the scale | 20 | 0 | 20 | 0 | |||||||
|
Patients with increase in bacterial |
5 | +10 | 12 | +21 | |||||||
| Patient death | 1* | +7 | 0 | 0 | |||||||
| * Death determined to be not related to study drug, data handling rules require any death to be scored as a +7 | |||||||||||
|
ARIKAYCE: TARGET NTM (TR02-112 STUDY): Overview of Adverse Events |
||||||||
|
ARIKAYCE |
Placebo |
Overall (N=89) |
||||||
| Number (%) of subjects with treatment-emergent adverse events | 41 (93.2) | 40 (88.9) | 81 (91.0) | |||||
| Number of treatment-emergent adverse events | 240 | 140 | 380 | |||||
| Number (%) of subjects with treatment-emergent adverse events by maximum severity | ||||||||
| Grade 1: Mild | 12 (27.3) | 25 (55.6) | 37 (41.6) | |||||
| Grade 2: Moderate | 24 (54.5) | 10 (22.2) | 34 (38.2) | |||||
| Grade 3: Severe | 4 (9.1) | 5 (11.1) | 9 (10.1) | |||||
| Grade 4: Life Threatening or Disabling | 0 | 0 | 0 | |||||
| Grade 5: Death | 1* (2.3) | 0 | 1 (1.1) | |||||
| Number (%) of subjects with treatment-emergent adverse events by seriousness | ||||||||
| Serious | 8 (18.2) | 4 (8.9) | 12 (13.5) | |||||
| Not Serious | 33 (75.0) | 36 (80.0) | 69 (77.5) | |||||
| Number of treatment-emergent serious adverse events | 12 | 5 | 17 | |||||
| Number (%) of subjects with treatment-emergent adverse events by strongest relationship to study drug [1] | ||||||||
| Related | 32 (72.7) | 17 (37.8) | 49 (55.1) | |||||
| Not Related | 9 (20.5) | 23 (51.1) | 32 (36.0) | |||||
| Number of subjects who discontinued | 9 | 0 | 9 | |||||
| * Death determined to be not related to study drug | ||||||||