EXTON, Pa.--(BUSINESS WIRE)--Lungpacer Medical, Inc. today announced results from the RESCUE 2 multicenter, randomized, controlled trial presented at the European Respiratory Society (ERS) 2020 Virtual International Congress. The RESCUE 2 trial compared Lungpacer® therapy plus Standard of Care (SoC) vs. SoC alone in patients who are receiving prolonged invasive mechanical ventilation (MV). RESCUE 2 demonstrated that Lungpacer therapy improves diaphragm strength by an average of 246% compared to SoC alone, as measured by improvement in Maximal Inspiratory Pressure (MIP).1 Improvement in MIP has been shown to correlate with weaning success in MV patients2,3 and is associated with lower mortality.2,4 The Lungpacer therapy group also showed a strong positive trend in proportion of MV patients weaned and reduction in average MV duration, as compared to SoC.1
While MV is a life-saving therapy, unfortunately, it comes with unwanted side effects. Ventilators use positive pressure to push air into the lungs, leaving the diaphragm unused and causing it to weaken quickly. The Lungpacer System is designed to stimulate the diaphragm during MV and strengthen the weakened muscle, so that the patient can be weaned off MV and resume natural breathing. The Lungpacer System is in the midst of clinical studies in the U.S. and Europe and has been authorized for emergency use by the FDA for patients on invasive mechanical ventilation who are at high risk of weaning failure during the COVID-19 pandemic. There are currently no FDA approved products to assist patients in weaning from mechanical ventilation.
“Typically to remove a patient from invasive mechanical ventilation, we have relied on spontaneous breathing trials or experience to see if the patient is ready to wean. These trials involve removing the patient from the ventilator and closely observing the patient’s success at breathing independently. The results of the RESCUE 2 study showed that the Lungpacer System improved MIP, an indicator of diaphragm strengthening, and also showed a positive trend for weaning success. Lungpacer therapy may, therefore, offer our ventilated patients a better chance at breathing independently again,” noted Dr. Martin Dres, Respiratory and Critical Care Department, APHP, Sorbonne University.
“The RESCUE 2 patient population had already been on invasive MV for an average of 25.2 days, making them at high-risk for MV weaning failure and Ventilator-Induced Diaphragm Dysfunction (VIDD). The MIP improvement results, combined with reduction in average MV days, suggests that Lungpacer therapy may offer hospital ICUs another option for improving outcomes for MV patients,” said Doug Evans, CEO of Lungpacer Medical, Inc. “Our therapy may also allow hospitals to free up precious ICU beds and resources, enabling them to better serve their patient population, particularly during the coronavirus pandemic.”
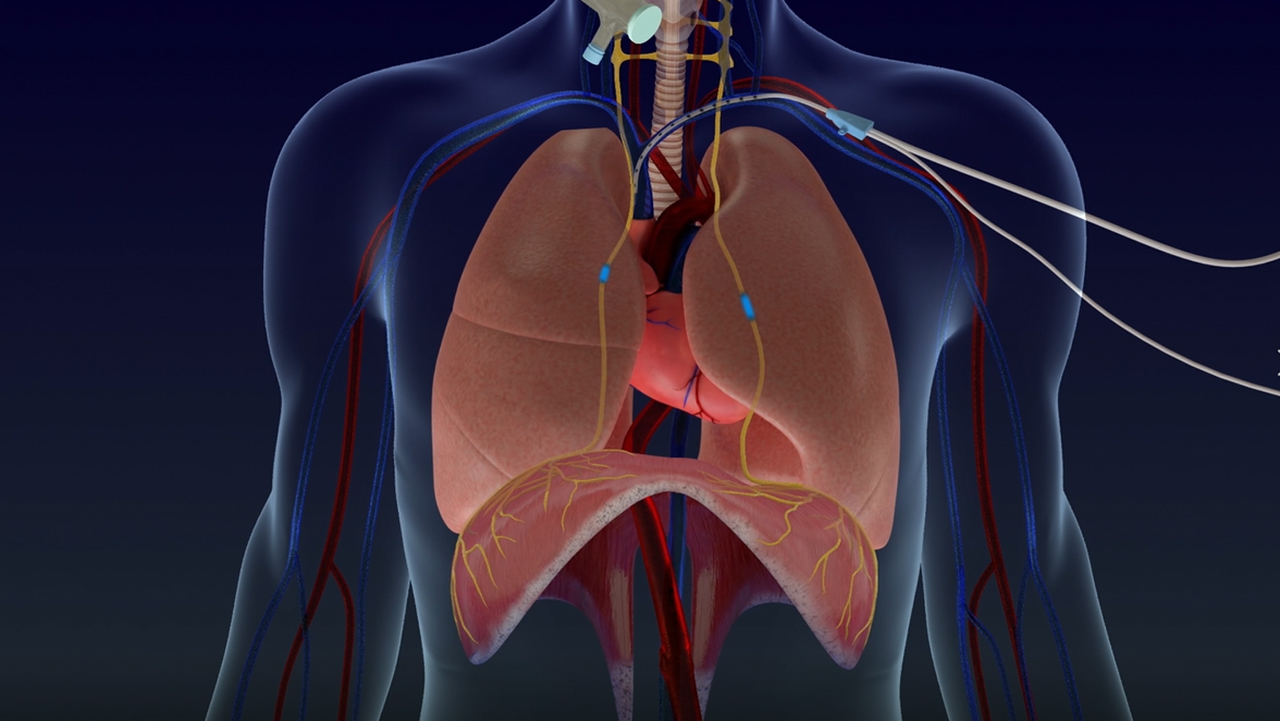
The Lungpacer System is designed to fit into the routine care of mechanically ventilated patients. The System uses a catheter (LIVE Catheter®), similar to the central venous catheters placed in most ventilated patients. This catheter can deliver both fluid and medications as well as stimulate the phrenic nerve to contract the diaphragm, exercising the muscle.
The RESCUE 2 results also help to validate the design of the RESCUE 3 pivotal clinical trial, currently underway at multiple sites in Europe and the United States. RESCUE 3 is designed to determine statistically significant weaning success in patients treated with Lungpacer therapy plus SoC vs. SoC alone in patients who are receiving prolonged invasive mechanical ventilation.
About Mechanical Ventilation and the COVID-19 Pandemic
According to the World Health Organization (WHO), severe COVID-19 patients commonly suffer from severe pneumonia, sepsis and Acute Respiratory Distress Syndrome (ARDS); these patients may require 15 to 20 days of mechanical ventilation during their treatment.4 The WHO guideline for management of these patients to preserve lung function includes methods to reduce complications related to mechanical ventilation such as reducing the time on MV and decreasing the incidence of ventilator-associated pneumonia.
About the Lungpacer® System
The Lungpacer System is a novel, first-in-class, temporary transvenous diaphragm neurostimulation system, FDA-designated as a breakthrough device, with FDA Emergency Use Authorization (EUA) during the COVID-19 pandemic. The Lungpacer System operates using the following main components: Lungpacer IntraVenous Electrode - LIVE Catheter®, Lungpacer Control Unit, and Intermediate Cable. The LIVE Catheter is a minimally invasive, central venous catheter used to deliver fluids and medications and to activate the diaphragm muscle via transvenous phrenic-nerve stimulation. The Lungpacer Control Unit is a mobile, portable unit that is used with the LIVE Catheter and Intermediate Cable to provide temporary transvenous diaphragmatic neurostimulation.
About the Emergency Use Authorization
On April 14, 2020 the FDA authorized the emergency use of the Lungpacer Diaphragm Pacing Therapy System™ for “patients on invasive mechanical ventilators at high risk of weaning failure, including COVID-19 patients requiring ventilation and patients being mechanically ventilated for other high-risk conditions including post-cardiac and post-thoracic surgical procedures and medical ICU patients requiring prolonged ventilation.6 Based on pre-clinical and clinical experience, FDA has concluded that the Lungpacer System may be effective at treating patients during COVID-19 by helping wean them off ventilators in healthcare settings, thereby reducing their risks of prolonged MV and increasing the availability of ventilators during the COVID-19 pandemic. The scope of the Emergency Use Authorization is limited to the use of the Lungpacer System for emergency use to assist in weaning patients determined by their healthcare provider to be at high risk of weaning failure off of ventilators in healthcare settings during the COVID-19 pandemic, for a maximum treatment duration of 30 days.
For more information on the Lungpacer System, please visit: www.lungpacer.com
- Dres, at al. Neurostimulation in difficult-to-wean mechanically ventilated patients - Results of the RESCUE 2 Randomized Controlled Trial. Poster presented at ERS International Congress 2020 - Virtual, August 2020.
- Wunsch H. Mechanical Ventilation in COVID-19: Interpreting the Current Epidemiology [published online ahead of print, 2020 May 13]. Am J Respir Crit Care Med. 2020;10.1164/rccm. 202004-1385ED. doi:10.1164/rccm.202004-1385ED.
- Kaier, K, Heister, T, Motschall, E, Hehn, P, Bluhmki, T Wolkewitz, M,; Impact of MV on the daily costs of ICU costs: A systematic review and meta regression; Epidemiol Infect. 2019 Dec 5;147:e314. doi: 10.1017/S0950268819001900.
- Historical Data Collection for Subjects Requiring a Prolonged Wean from Invasive Mechanical Ventilation, On MV ≥ 7 Days and failed to wean, Temple University Hospital, Sponsor: Lungpacer Medical, Inc. 2016.
- Rosenbaum, R. “Facing Covid-19 in Italy - Ethics, Logistics, and Therapeutics on the Epidemic’s Front Line.” N Engl J Med, Published online March 19, 2020 DOI: 10.1056/NEJMp2005492.
- FDA Emergency Use Authorization Letter (April 14, 2020).
EMERGENCY USE INDICATION: The Lungpacer DPTS has been authorized for emergency use in healthcare settings for treatment of patients on invasive mechanical ventilation who are at high risk of weaning failure, including COVID-19 patients, during the COVID-19 pandemic. The Lungpacer DPTS may improve inspiratory muscle strength and weaning success in patients ages 18 years or older who have failed to wean from mechanical ventilation.
IMPORTANT SAFETY AND EFFECTIVENESS INFORMATION: There are no FDA approved, licensed, or cleared device treatments to assist in weaning patients off of ventilators. Based on bench testing and reported clinical experience, FDA has concluded that the Lungpacer DPTS may be effective at treating patients during COVID-19 by helping wean patients off ventilators in healthcare settings, thereby reducing their risks of prolonged mechanical ventilation and increasing the availability of ventilators during the COVID-19 pandemic.
Lungpacer DPTS treatment is for a maximum of 30 days. Device-related serious adverse events reported in clinical trials were single events of pneumothorax and mucus plug liberation. The LIVE Catheter component of the Lungpacer system has not been evaluated for safety when used with cardiac pacemakers or defibrillators and should be removed prior to Magnetic Resonance (MR) imaging. Do not place the LIVE catheter into or allow it to remain in the right atrium or right ventricle.
Emergency Use Instructions for Use, Fact Sheet for Healthcare Providers, and Fact Sheet for Patients are available at: http://lungpacer.com/emergency-use-authorization/
The Lungpacer DPTS is available under an Emergency Use Authorization for the duration of the COVID-19 pandemic. For more information visit https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations.
EMERGENCY USE AUTHORIZATION INFORMATION: This Diaphragmatic Pacing Stimulator has not been FDA cleared or approved. This Diaphragmatic Pacing Stimulator has been authorized by FDA under an Emergency Use Authorization (EUA). This Diaphragmatic Pacing Stimulator is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of Diaphragmatic Pacing Stimulator Systems under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Lungpacer®, Weaning Solution®, Lungpacer DPT System™, Lungpacer DPT™, LIVE Catheter® are registered trademarks of Lungpacer Medical.
Copyright © 2020. All rights reserved.
MKT-0000, RevA 082020