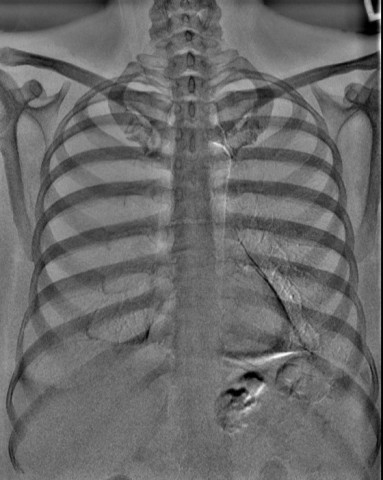
ROCHESTER, N.Y.--(BUSINESS WIRE)--Carestream’s Dual-Energy imaging technology and its Focus 35C Detector with Image Suite Software have received 510(k) clearance from the U.S. Food and Drug Administration. Both technologies will be showcased at Carestream’s booth #7513 at the upcoming Radiological Society of North America annual conference.
Carestream’s Dual-Energy application—powered by the company’s Eclipse image processing engine—utilizes two filter materials that are automatically switched between the high- and low-energy exposures to produce a soft-tissue-only image with the bone structures removed, as well as a corresponding bone-only image. This differential filter approach optimizes both X-ray spectrums, achieving optimal dose efficiency. It also delivers outstanding image quality—at the equivalent patient exposure as a standard, non-Dual-Energy posterior-anterior chest radiograph. Carestream’s Dual-Energy technology has been approved for chest X-rays on its DRX-Evolution Plus System.
“When performing a standard chest X-ray, you see both soft tissue and bone structures overlaying on top of each other,” said Sarah Verna, Worldwide Marketing Manager for Global X-ray Solutions at Carestream. “Dual-Energy will help radiologists improve the specificity and sensitivity for chest diagnosis because of both material differentiation and the removal of overlapping anatomical structures.”
Dual-Energy technology is another example of Carestream’s ability to use algorithmic results to provide better medical image quality and improve diagnostic capabilities, while keeping patient safety in mind.
“This technology takes two images in rapid succession but when you compare the total Entrance Skin Exposure to the patient, it’s the same as a standard PA chest exam. Dual-Energy does not expose the patient to more radiation,” Ms. Verna added.
Carestream’s new Focus 35C Detector with Image Suite Software offers smaller facilities and specialty practices a budget-friendly way to tap the power of digital medical imaging. This highly economical retrofit solution combines advanced image processing with broad functionality, easily transforming an analog X-ray room into a full wireless digital radiography system. The Focus 35C Detector paired with Image Suite Software provide a mini Picture Archiving and Communication System (PACS), delivering a complete imaging package so the customer benefits from all the capabilities of a PACS without having to invest in all the features of a larger system.
Carestream’s Focus 35C Detector is expected to be commercially available by the end of the year, while the company’s Dual-Energy technology is expected to be released in early 2020.
About Carestream Health
Carestream is a worldwide provider of medical imaging systems; X-ray imaging systems for non-destructive testing; and precision contract coating services for a wide range of industrial, medical, electronic and other applications—all backed by a global service and support network. For more information about the company’s broad portfolio of products, solutions and services, please contact your Carestream representative or call 1-888-777-2072 or visit www.carestream.com.
CARESTREAM is a trademark of Carestream Health.
Follow Carestream Health online:
http://www.twitter.com/carestream
http://www.youtube.com/carestream
http://www.carestream.com/blog/
http://www.facebook.com/carestream
http://www.linkedin.com/company/carestream-health
https://www.instagram.com/carestreamhealth/
“Rx only”
2019