EL SEGUNDO, Calif.--(BUSINESS WIRE)--The KARL STORZ Photodynamic Diagnosis (PDD) System - Blue Light Cystoscopy with Cysview (BLCC) is one of the medical innovations that was showcased in front of thousands of healthcare providers at Premier Inc.’s 2017 Breakthroughs Conference and Exhibition on June 27.
Blue Light Cystoscopy with Cysview using KARL STORZ PDD technology, aimed at early detection of non-muscle invasive bladder cancer (NMIBC), was debuted during the conference’s annual Innovation Celebration. The event recognizes advances in healthcare while highlighting industry suppliers committed to innovation and improving patient outcomes.
“KARL STORZ shares Premier’s commitment to providing valuable products to our alliance members that are safe, high-quality and cost-effective,” said Durral R. Gilbert, president of supply chain services, Premier. “We believe these innovations can benefit providers as they work to transform healthcare.”
Clinicians and other health system members of Premier selected BLCC technology to be showcased at the Innovation Celebration due to its uniqueness, ability to have an impact on unmet clinical needs and potential to improve patient care.
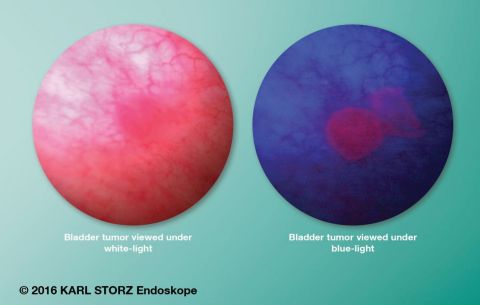
The KARL STORZ PDD system enhances detection and treatment of NMIBC. The PDD system uses a photosensitive marker, Cysview (a registered trademark of Photocure, ASA), which is introduced into the bladder, absorbed and converted into a pigment that is deposited into cancerous tissue. At the heart of this system is the KARL STORZ D-LIGHT C light source as well as specially adapted telescopes. Under blue light excitation, this pigment emits red fluorescence while normal tissue appears dark blue. This helps the physician to clearly identify tumorous tissue. It is the only FDA-approved technology that is clinically proven to detect more Ta/T1 bladder cancer lesions vs. white-light cystoscopy alone, and promote a more comprehensive tumor resection. BLCC is recommended to decrease recurrence of bladder cancer and avoid complications associated with TURBT.
“Use of BLCC offers significant new diagnostic and treatment capabilities to Healthcare providers striving to offer state-of-the-art care to their patients and promoting improved clinical outcomes,” says Les Friend, Executive Director, Corporate Accounts, KARL STORZ. “In fact, the American Urological Association and Society for Urologic Oncology included Blue Light Cystoscopy in the 2016 Guidelines for the management of NMIBC.”
He adds, “We consider it an honor to support the world-class level of care and treatment that the Clinical teams offer to their patients and to showcase this technology as one of the 2017 Masters of Innovation.”
Any supplier, regardless of whether the company is contracted with Premier, can be considered for participation in the Innovation Celebration. Premier contracts with more than 1,200 suppliers.
For more detailed information, including a video of Blue Light Cystoscopy with Cysview, visit: http://breakthroughs.premierinc.com/innovation-celebration-2017/.
About Premier Inc.
Premier Inc. is a leading healthcare improvement company, uniting an alliance of approximately 3,750 U.S. hospitals and more than 130,000 other provider organizations. With integrated data and analytics, collaboratives, supply chain solutions, and advisory and other services, Premier enables better care and outcomes at a lower cost. Please visit Premier’s news and investor sites on www.premierinc.com.
About KARL STORZ
KARL STORZ Endoscopy-America, Inc., is an affiliate of KARL STORZ GmbH & Co. KG, an international leader for more than 70 years in reusable endoscope technology, encompassing all endoscopic specialties. Based in Tuttlingen, Germany, KARL STORZ GmbH & Co. KG is a family-owned company that designs, engineers, manufactures, and markets all its products with an emphasis on visionary design, precision craftsmanship and clinical effectiveness. For more information, call (800) 421-0837 or visit the company’s Web site at www.karlstorz.com.