CHIGAGO--(BUSINESS WIRE)--Connecticut-based medical device innovator Lumendi, LLC (www.lumendi.com) has announced its new endoscopic accessory, DiLumen™ EIP, is now available for use as indicated to ensure complete positioning of an endoscope in the large intestine and assist with optical visualization, diagnosis and endoscopic treatment. Because such a device may improve endolumenal treatments, DiLumen™ EIP may better enable these less invasive procedures and take the place of open or laparoscopic surgery, thereby preserving anatomy, shortening recovery and reducing healthcare costs.
The announcement was made at the annual meeting of Digestive Disease Week (DDW) in Chicago, Illinois, at which Lumendi exhibited and provided in-booth demonstrations of the clinical versatility and value of DiLumen™. Lumendi received U.S. FDA 510(k) clearance to market DiLumen™ in December 2016. A post-market outcome study is now underway to demonstrate the capabilities of DiLumen during endoscopy procedures.
“DiLumen is an important advance in a field defined until now by laparoscopic and open surgical procedures. We recently hired a team of five dedicated regional sales managers, each of whom has extensive experience in medical devices and new technology as well as gastroenterology and related diseases. This team will help educate and train advanced endoscopists, colorectal surgeons and gastroenterologists nationwide,” said Dr. Peter Johann, CEO of Lumendi, Ltd.
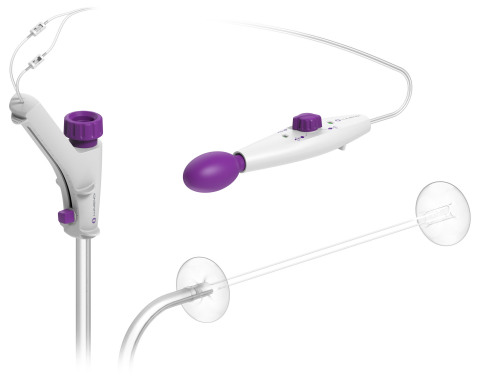
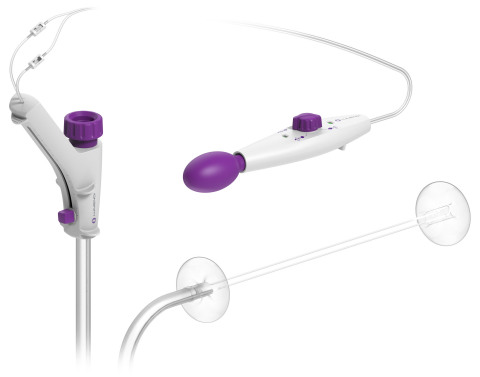
The device fits over a flexible endoscope and adds additional stabilization and improved visualization during incision-free endolumenal therapeutic procedures. Endolumenal treatment is different because it provides access to the therapeutic area within the colon through the anus. Because the procedure and treatment take place entirely within the intestine and there are no incisions through the skin, recovery time is reduced and the colon is preserved.
DiLumen consists of a single-use, soft flexible sheath that fits over standard and small-diameter endoscopes. The device employs two balloons, one behind the bending section of the endoscope and the second in front of the tip of the endoscope. When both balloons are deployed, and inflated, a stable Therapeutic Zone™ (TZ) is created. This TZ facilitates more localized insufflation and manipulation of the colon and provides improved access to lesions to enable endoscopists and surgeons to perform precise endolumenal interventions. Once the procedure is complete, the balloons are deflated and removed along with the endoscope.
About Lumendi, LLC http://www.lumendi.com Headquartered in Westport, Connecticut, Lumendi, LLC is a wholly owned subsidiary of Lumendi, Ltd., a privately held innovative, medical device company headquartered in London, England. Lumendi is focused on developing, marketing and distributing surgical tools and devices that provide safe, cost-effective solutions for minimally invasive gastrointestinal interventions. Lumendi Ltd. holds a worldwide exclusive license from Cornell University on the DiLumen technology.