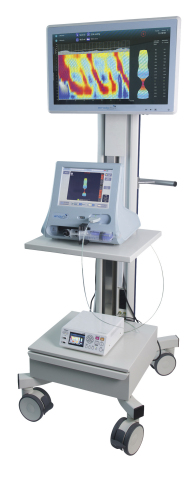
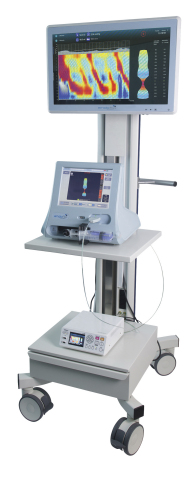
GALWAY, Ireland--(BUSINESS WIRE)--Crospon, an endoscopic diagnostics company, today announced it has received US Food and Drug Administration (FDA) 510(k) clearance for its Endoflip® System with Flip® Topography Module, providing a new way for clinicians to assess patient motility disorders during endoscopy.
Continuing Crospon’s commitment to deliver information into the hands of gastroenterologists, the next generation of the Endoflip® System, Endoflip® 2.0, introduces imaging software that displays real-time esophageal contractility patterns on a 24” touch-screen. The unique repetitive visual cues of the patented Endoflip® technology enables gastroenterologists, for the first time, to investigate for conditions such as achalasia, GEJ outflow obstruction and other major or minor disorders of peristalsis during endoscopy. EndoFLIP 2.0 also introduces the new capability to capture and store Endoflip® images in electronic patient records.
“This clearance represents a significant milestone for our organisation as we can now offer physicians an additional tool that can be used during endoscopy, along with other diagnostic methods, to evaluate patients with symptoms consistent with gastrointestinal motility disorders”, said John O’Dea, CEO and Founder of Crospon.
“The stand out benefit of this application, for both patients and caregivers, is that the Flip® Topography assessment can be performed in less than five minutes during upper endoscopy on a sedated patient. In addition to significantly reducing discomfort, the patient may not have to wait for testing at a specialised center, thereby reducing their time to treatment. While not always a substitute for manometry, Endoflip® 2.0 can reveal situations of non-obstructive dysphagia which may benefit from further evaluation from manometry.”
Shipments of Endoflip® 2.0 systems will commence in June 2017. For more information, please visit crospon.com.
Ends:
About Crospon
Crospon is an endoscopic diagnostics company that is changing the face of esophageal function testing with its Endoflip® and Esoflip® technologies. The minimally invasive medical devices transform the patient experience when it comes to swallow testing and deliver a whole new diagnostic tool set into the hands of gastroenterology professionals.
Established in 2006 by chairman and chief executive John O’Dea and with offices in the US and Ireland, Crospon’s experienced team designs and manufactures medical devices that allow clinicians to assess the real-time effects of their interventions during endoscopic and laparoscopic procedures.