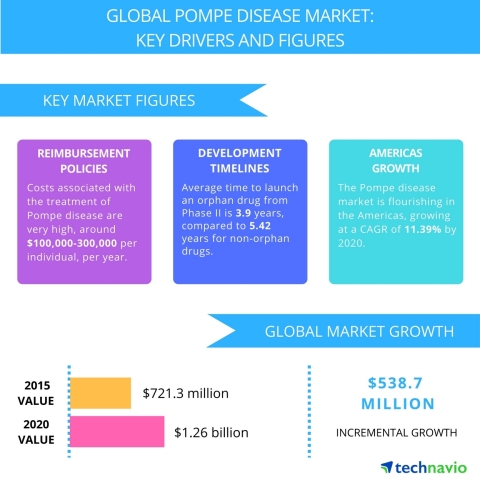
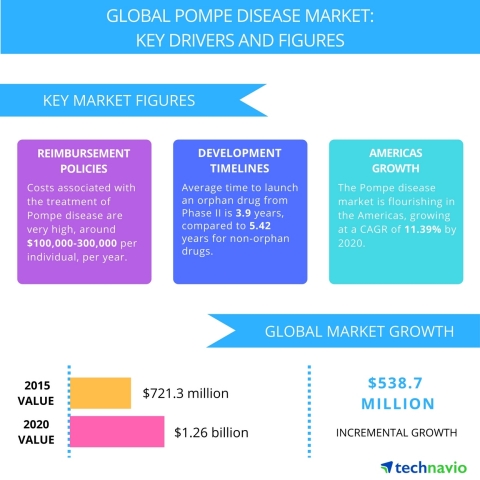
LONDON--(BUSINESS WIRE)--Technavio analysts forecast the global Pompe disease treatment market to grow at a CAGR of almost 12% during the forecast period, according to their latest report.
The research study covers the present scenario and growth prospects of the global Pompe disease treatment market for 2016-2020. To calculate the market size, the report considers revenue from the sales of the drugs available in the market and the drug candidates that are in various phases of development for the treatment of Pompe disease.
The regulatory assistance provided to drug manufacturers in emerging markets is expected to propel the growth of the market during the forecast period. Developing economies are growingly becoming aware of the significance of orphan drug designations and are working toward defining the regulatory protocols required to award these designations. In addition, these countries are also working on providing incentives such as reimbursements to pharmaceutical manufacturers to encourage research in this area.
Request a sample report: http://www.technavio.com/request-a-sample?report=55145
Technavio’s sample reports are free of charge and contain multiple sections of the report including the market size and forecast, drivers, challenges, trends, and more.
Technavio healthcare and life sciences analysts highlight the following four factors that are contributing to the growth of the global Pompe disease treatment market:
- Special regulatory drug designations for orphan drugs
- Reimbursement policies for treatment
- Designated under ICD-10 code
- Shorter development timelines
Special regulatory drug designations for orphan drugs
Pompe disease is listed as a rare disease by regulatory authorities of the US and Europe. Diseases with a limited patient population mean a smaller market for treatment, which might not be profitable in terms of recovering the R&D expenses of the drug. To overcome this limitation, the US and the EU have provisions for orphan drug designation for drugs developed to treat Pompe disease.
Sapna Jha, a lead central nervous system research analyst at Technavio, says, “In the EU, orphan drugs receive protocol assistance and 10-year market exclusivity. In the US, they are offered incentives such as tax credits for clinical testing, exemption from prescription fees, and market exclusivity for seven years.”
Reimbursement policies for treatment
The costs associated with the treatment of Pompe disease are very high, USD 100,000-300,000 per individual per annum, making it difficult for individuals to continue with the treatment for a long period. According to Genzyme report 2015, an estimated the treatment cost of an adult and a child with Myozyme to be USD 300,000 per annum and about USD 100,000 per year, respectively.
“Organizations such as the Canadian Association of Pompe, Acid Maltase Deficiency Association, and International Pompe Association provide financial support to individuals to continue the therapy. The existence of such programs is expected to drive the growth of the market during the forecast period,” adds Sapna.
Designated under ICD-10 code
International classification of disease (ICD) is a standard tool used globally for analytical and diagnostic purposes. Under this, the codes are provided to the individuals to emphasize the need to visit medical centers and by healthcare partners to understand the frequency of medical visits recommended for the individuals. Thus, ICD codes can be used for reimbursement purposes by private and government insurers.
Shorter development timelines
The shorter development timelines are anticipated to have a positive impact on the market for orphan drugs during the forecast period. The clinical trials of orphan drugs are significantly shorter than those of non-orphan drugs owing to the special status. The average time required to launch an orphan drug from Phase II is 3.9 years, compared with 5.42 years for the non-orphan drugs.
Top vendors:
- Amicus Therapeutics
- Audentes Therapeutics
- Sanofi Genzyme
Browse Related Reports:
- Global Human Respiratory Syncytial Virus Drugs Market 2016-2020
- Global Alopecia Drugs Market 2016-2020
- Pompe Disease Market in the US 2015-2019
Become a Technavio Insights member and access all three of these reports for a fraction of their original cost. As a Technavio Insights member, you will have immediate access to new reports as they’re published in addition to all 6,000+ existing reports covering segments like cardiovascular devices, oncology, and in-vitro diagnostics. This subscription nets you thousands in savings, while staying connected to Technavio’s constant transforming research library, helping you make informed business decisions more efficiently.
About Technavio
Technavio is a leading global technology research and advisory company. The company develops over 2000 pieces of research every year, covering more than 500 technologies across 80 countries. Technavio has about 300 analysts globally who specialize in customized consulting and business research assignments across the latest leading edge technologies.
Technavio analysts employ primary as well as secondary research techniques to ascertain the size and vendor landscape in a range of markets. Analysts obtain information using a combination of bottom-up and top-down approaches, besides using in-house market modeling tools and proprietary databases. They corroborate this data with the data obtained from various market participants and stakeholders across the value chain, including vendors, service providers, distributors, re-sellers, and end-users.
If you are interested in more information, please contact our media team at media@technavio.com.