NEW YORK--(BUSINESS WIRE)--Kadmon Holdings, Inc. (NYSE: KDMN) (“Kadmon” or the “Company”) today announced encouraging data from its ongoing Phase 2 clinical study of tesevatinib, the Company’s blood-brain barrier penetrant oral tyrosine kinase inhibitor, for the treatment of epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC) that has metastasized to the brain and/or the leptomeninges (membranes lining the brain and spinal cord). The data were presented today in a poster session at the International Association for the Study of Lung Cancer 17th World Conference on Lung Cancer in Vienna, Austria.
Eleven of the first 13 enrolled patients treated with tesevatinib 300 mg QD in this ongoing study did not have central nervous system (CNS) progression on tesevatinib. Eight of these patients showed an improvement in clinical symptoms, often by Day 14 of treatment. Intracranial radiological improvement was documented in four patients who had follow-up MRIs.
The study is being conducted in patients who have progressed with brain metastases and/or symptomatic leptomeningeal metastases while on prior therapy with other EGFR inhibitors, as well as in patients with no prior treatment and brain metastases at initial presentation. Of the 13 enrolled patients, 12 had disease progression while on prior treatment with the EGFR inhibitor erlotinib and radiation therapy to the brain, five of whom had also received other EGFR inhibitors and chemotherapy. Currently approved EGFR inhibitors have poor brain penetration, limiting their ability to treat intracranial metastases. The rapid action of tesevatinib on clinical symptoms and shrinkage of tumor volumes demonstrates conclusively that tesevatinib enters the CNS and targets EGFR-driven tumors.
The study was designed specifically to assess the efficacy of tesevatinib in CNS metastases, with full knowledge that these heavily pretreated patients had extensive exposure to other EGFR inhibitors and that tesevatinib therefore may not control peripheral disease well due to the previous development of EGFR inhibitor resistance mechanisms. Thus, as expected, five of the 12 pretreated patients had peripheral disease progression, while in four of those five patients, tesevatinib controlled CNS lesions.
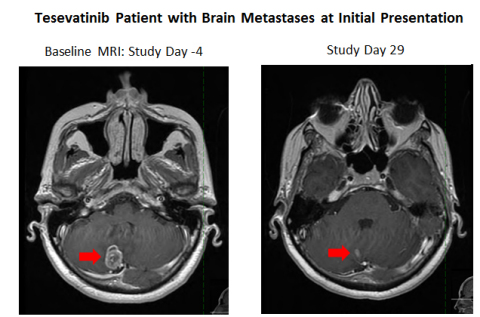
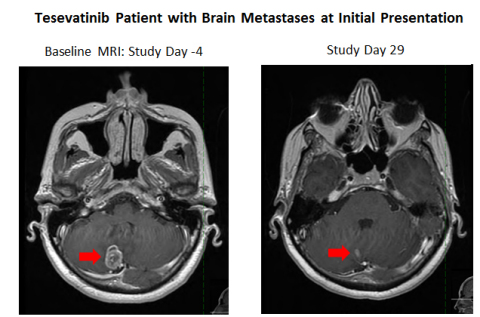
In addition to the results observed in pretreated patients, one enrolled patient with no prior treatment who presented with brain metastases showed a robust partial response in brain metastases in an MRI taken on Study Day 29 and showed a partial response in both brain metastases and peripheral disease at Study Day 57. This patient continues on tesevatinib as of Study Day 92. These findings support the notion that tesevatinib monotherapy has the potential to be a first-line treatment in this patient population.
Based on these interim results, Kadmon plans to initiate a randomized, first-line study of tesevatinib monotherapy in patients with EGFR-mutant NSCLC who present with CNS metastases. In addition, Kadmon will enroll a randomized study of tesevatinib in patients with leptomeningeal metastases who have progressed while on currently approved EGFR inhibitor therapies.
“Unlike other EGFR inhibitors, tesevatinib penetrates the blood-brain barrier to reach metastases in the central nervous system, a sanctuary site for EGFR-driven cancers,” said David Berz, M.D., Ph.D., MPH, clinical oncologist at Beverly Hills Cancer Center and a principal investigator of the study. “These results indicate that tesevatinib has major therapeutic potential for CNS metastases in the first-line setting as well as in heavily pretreated patients.”
“The dramatic responses observed in a high proportion of these NSCLC patients with intracranial metastases, particularly in difficult-to-treat individuals, support our continued development of tesevatinib for the treatment of metastatic NSCLC,” said Harlan W. Waksal, M.D., President and Chief Executive Officer at Kadmon. “Based on these encouraging initial results, we believe that tesevatinib as first-line therapy may treat existing CNS metastases as well as potentially prevent the development of new lesions.”
About Kadmon Holdings, Inc.
Kadmon Holdings, Inc. is a fully integrated biopharmaceutical company focused on developing innovative products for significant unmet medical needs. We have a diversified product pipeline in oncology, autoimmune and fibrotic diseases and genetic diseases.
Safe Harbor Statement
This press release contains forward-looking statements. Such statements may be preceded by the words “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “targets,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. We believe that these factors include, but are not limited to, (i) the initiation, timing, progress and results of our preclinical studies and clinical trials, and our research and development programs; (ii) our ability to advance product candidates into, and successfully complete, clinical trials; (iii) our reliance on the success of our product candidates; (iv) the timing or likelihood of regulatory filings and approvals; (v) our ability to expand our sales and marketing capabilities; (vi) the commercialization of our product candidates, if approved; (vii) the pricing and reimbursement of our product candidates, if approved; (viii) the implementation of our business model, strategic plans for our business, product candidates and technology; (ix) the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and technology; (x) our ability to operate our business without infringing the intellectual property rights and proprietary technology of third parties; (xi) costs associated with defending intellectual property infringement, product liability and other claims; (xii) regulatory developments in the United States, Europe and other jurisdictions; (xiii) estimates of our expenses, future revenues, capital requirements and our needs for additional financing; (xiv) the potential benefits of strategic collaboration agreements and our ability to enter into strategic arrangements; (xv) our ability to maintain and establish collaborations or obtain additional grant funding; (xvi) the rate and degree of market acceptance of our product candidates; (xvii) developments relating to our competitors and our industry, including competing therapies; (xviii) our ability to effectively manage our anticipated growth; (xix) our ability to attract and retain qualified employees and key personnel; and (xx) our ability to achieve cost savings and other benefits from our efforts to streamline our operations and to not harm our business with such efforts. More detailed information about Kadmon and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's filings with the U.S. Securities and Exchange Commission (SEC), including the Company's Quarterly Report on Form 10-Q filed pursuant to Section 13 of the Securities Exchange Act of 1934, as amended, with the SEC on November 9, 2016. Investors and security holders are urged to read these documents free of charge on the SEC's web site at www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future events or otherwise.