NORTH WALES, Pa.--(BUSINESS WIRE)--Teva Parenteral Medicines today announced a voluntary recall of six lots of Adrucil® (fluorouracil injection, USP) 5 g/100 mL (50 mg/mL) due to the potential presence of particulate matter identified as aggregate of silicone rubber pieces from a filler diaphragm and fluorouracil crystals. The recalled lots are as follows:
| Lot # | Exp. Date | Vial Size | NDC# individual vials | NDC# carton of 5 vials | ||||||||
| 31317857B | 8/2015 | 100 mL | 0703-3019-11 | 0703-3019-12 | ||||||||
| 31317859B | 12/2015 | 100 mL | 0703-3019-11 | 0703-3019-12 | ||||||||
| 31317920B | 12/2015 | 100 mL | 0703-3019-11 | 0703-3019-12 | ||||||||
| 31317957B | 12/2015 | 100 mL | 0703-3019-11 | 0703-3019-12 | ||||||||
| 31318136B | 12/2015 | 100 mL | 0703-3019-11 | 0703-3019-12 | ||||||||
| 31318138B | 12/2015 | 100 mL | 0703-3019-11 | 0703-3019-12 | ||||||||
Administration of an intravenous product with particulate matter has the potential to result in inflammation, allergic reactions, or blockage of blood vessels, leading to tissue death, which may be life-threatening if vital organs are affected. To date, Teva has not received any reports of adverse events related to this recall.
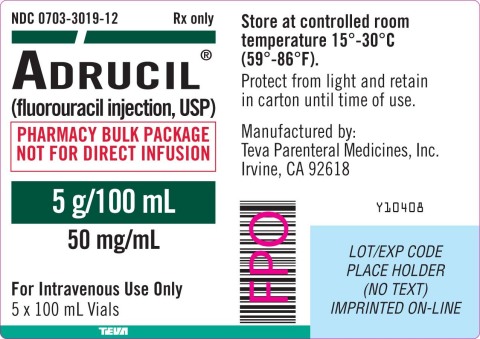
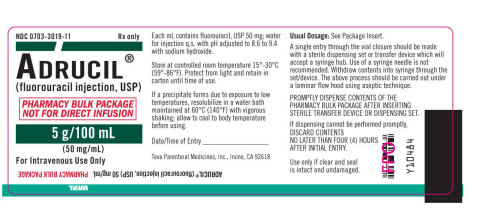
Adrucil® Injection is used in the palliative management of carcinoma of the colon, rectum, breast, stomach and pancreas and is packaged in pharmacy bulk packages. The pharmacy bulk package has five 5 g/100ml vials per shelf pack. Individual Adrucil® 5 g/100 ml vials have the NDC code 0703-3019-11 and the pharmacy shelf pack has the NDC code 0703-3019-12. The Adrucil® 5 g/100 ml vial can be further identified by the statement on the label in red that states “PHARMACY BULK PACKAGE NOT FOR DIRECT INFUSION”. Adrucil® 5 g/100 ml vials were distributed in the United States. Teva has distributed this product nationwide through wholesalers, retailers, and pharmacies.
Teva has notified its direct customers by mail and has issued an Urgent Drug Recall Letter to direct customers. Teva is arranging for impacted product to be returned to Inmar. Anyone with an existing inventory of the recalled lots should stop use and distribution, and quarantine the product immediately. Customers should notify all users in their facility. Customers who have further distributed the recalled product should notify any accounts or additional locations which may have received the recalled product and instruct them if they have redistributed the product to notify their accounts, locations or facilities to the user level.
For medical related questions please contact Medical Information at 888-838-2872, option 3, then option 4. For a customer service related question, please contact Teva Customer Service at 800-545-8800 Monday – Friday; 8:00 – 5:00 EST. Consumers should immediately contact their physician or healthcare provider if they have experienced any problems that may be related to taking this drug product. Teva Parenteral Medicines is voluntarily recalling the aforementioned product lots with the knowledge of the U.S. Food and Drug Administration.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.