INGELHEIM, Germany--(BUSINESS WIRE)--For Non-US/Non-UK/Non-Canadian Media
New data demonstrate that a five-minute infusion of the investigational specific antidote idarucizumab led to immediate, complete and sustained reversal of the anticoagulant effect of Pradaxa® (dabigatran etexilate). In elderly individuals and those with mild or moderate kidney impairment, all doses of idarucizumab were found to be well tolerated. Dabigatran treatment could successfully be restarted 24 hours after the antidote had been administered, resulting in full re-anticoagulation.1 The new data were presented today at the 56th American Society of Hematology (ASH) Annual Meeting and Exposition, San Francisco, USA. The specific antidote to dabigatran is still under investigation and has not yet been approved for clinical use.
“These results showed that idarucizumab consistently reversed the anticoagulant effects of dabigatran in adults independent of gender, age and kidney function, complementing data previously reported from younger, healthy male volunteers,” said Professor Jörg Kreuzer, Vice President Medicine Therapeutic Area Cardiovascular, Boehringer Ingelheim. “Our findings show that the antidote’s effects remain consistent following a second administration and also that anticoagulation from dabigatran can be successfully restored 24 hours after idarucizumab is given.”
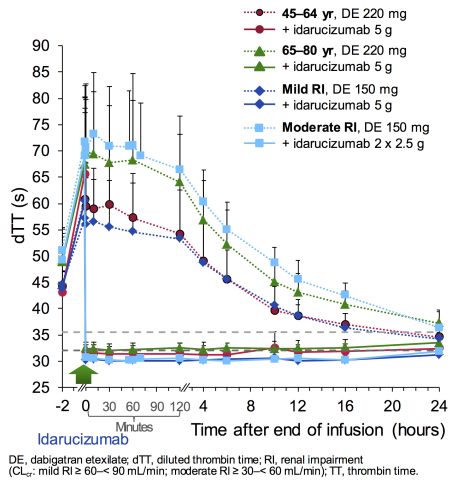
Idarucizumab, a humanized antibody fragment (Fab), was investigated in male and female healthy mid-aged adults (aged 45-64 years), elderly individuals (aged 65-80 years) and those with mild or moderate kidney impairment. The volunteers received dabigatran for 3.5 days before the specific antidote was administered. In some subjects, treatment with dabigatran was restarted 24 hours later. Measurements of the anticoagulant effect of dabigatran as well as its reversal by idarucizumab were taken at baseline, after administration of dabigatran, and after subsequent infusion of idarucizumab or placebo. After two months, the test was repeated in six healthy subjects.1
The findings from the new study showed:1
- Administration of idarucizumab resulted in immediate, complete and sustained reversal of dabigatran-induced anticoagulation in both age groups tested and also in subjects with reduced renal function
- Complete reversal was shown right at the end of the five minute infusion of idarucizumab
- Treatment with dabigatran could successfully be restarted 24 hours following administration of idarucizumab and resulted in full re-anticoagulation
- A second administration of idarucizumab, two months after the first one, achieved the same degree of reversal of dabigatran anticoagulation as the first administration
- Idarucizumab was well tolerated– no clinically relevant adverse events were reported
- For the 5g dose of idarucizumab, the reversal effect was consistent independent of age and kidney function. This dose is currently being investigated in the clinical setting3
“These latest data are important because this study included volunteers who were older and who had impaired kidney function – characteristics that are common among patients who require anticoagulation,” said Dr Georg van Husen, Head of Therapeutic Area Cardiovascular, Boehringer Ingelheim. “The growing evidence supports idarucizumab as a highly targeted antidote for dabigatran which can provide immediate, complete and sustained reversal. It would be an additional option for use in clinical situations where patients might benefit from a fast reversal of the anticoagulant effect of Pradaxa®.”
Boehringer Ingelheim is developing idarucizumab as a highly specific and selective antidote to Pradaxa® to add a reversal option for physicians. Idarucizumab was granted Breakthrough Therapy Designation by the U.S. Food and Drug Administration (FDA) in June 2014.2 RE-VERSE AD™, a global patient study, is investigating the reversal of anticoagulation in clinical situations where fast reversal is required. This is the first time that an antidote under development for a novel oral anticoagulant is being investigated in a study in patients, instead of volunteers.3
* Idarucizumab is the recommended International Nonproprietary Name (INN).
~ENDS~
Please click on the link below for ‘Notes to Editors’ and ‘References’: