TOKYO--(BUSINESS WIRE)--Philips Respironics GK (Headquartered in Minato Ward of Tokyo, hereafter Philips, CEO Danny Risberg) announced today that, beginning April 1, 2014, they are starting sales of a portable sleep diagnostic device WatchPAT™ (made by Itamar Medical Ltd., Caesarea, Israel) .
It is estimated that about three million potential patients are suffering from SAS (Sleep Apnea Syndrome) in Japan alone.
Currently, three sleep diagnostic methods are available in Japan with medical reimbursement. One of them is Itamar Medical’s WatchPAT™.
Philips is set to launch in Japan the new portable sleep diagnostic device WatchPAT™ that matches the accuracy and robustness of a full night PSG (polysomnography).
Overview and Features of WatchPAT®
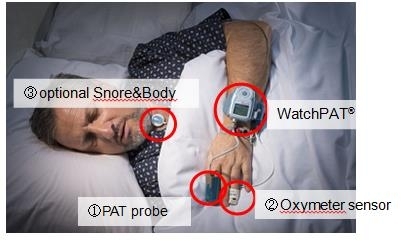
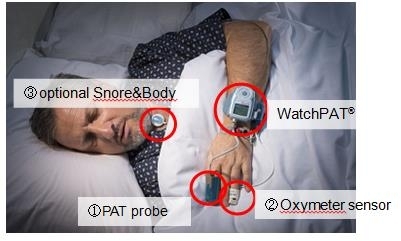
The WatchPAT™ portable sleep diagnostic device has three sensors that are attached to the patient’s body (see picture), allowing for comfortable sleep in a natural environment. Signals detected by each sensor are stored in the WatchPAT™’s internal memory and later downloaded to a PC with the zzzPAT™ software. The zzzPAT™ also generates a sleep study report.
-
WatchPAT™ is easy to handle, and provides accurate and
reliable diagnosis regardless of whether performed at home or at a
medical institution.
A patient can easily place the three sensors by him/herself. Home testing reduces the patients psychological, logistical, and economical concerns. Also, overall sleep is not affected by the harsh sleep lab or hospital conditions.
-
Three sensors including PAT® probe enable
an assessment of AHI and sleep stages.
The three recorded signals (PAT®, pulse oximetry and actigraphy) enable the accurate calculation of AHI by the proprietary zzzPAT™ algorithms. Unlike other home sleep devices, the WatchPAT™ differentiates between sleep and wake periods, utilizing the total sleep time rather than the total recording time and its AHI calculation better reflects the severity of the disorder. The sleep stage detection by the WatchPAT™ allows the physician to understand the implications of the disorder from the patient's sleep pattern and identify special conditions such as REM related SAS, thus taking it into consideration when deciding treatment.
About SAS (Sleep Apnea Syndrome)
SAS, or SDB (Sleep-Disordered Breathing), is a comprehensive term for ailments that cause abnormal breathing while sleeping. According to epidemiological data, the ratio of people who suffer from SDB is over 20% for men and 10% for women. The most common SDB is Obstructive Sleep Apnea Syndrome (OSAS). OSAS causes a sleeping person to halt breathing repeatedly due to the closing of the upper airway, leading to significant decrease of arterial oxygen saturation and multiple arousals. This condition reportedly increases the risk of various disorders such as hypertension, arrhythmia, ischemic heart disease, cerebral vascular disorder, diabetes, male impotency and sudden death. Also, repeated pauses in breathing cause arousals, excessive daytime sleepiness and cognitive impairment which may lead to traffic or labor accidents. Patients with OSAS take 1.7-1.9 times more medication for cardiovascular disease. Economic losses caused by sleeping disorders including OSAS are estimated to be about 3.4 trillion yen per year.
Symptoms of OSAS include snoring, excessive daytime sleepiness, daytime fatigue, depression, excessive urination at night and erectile dysfunction.
As obesity rate rises among Japanese people, the number of OSAS patients is expected to rise as well. Since 80 percent of those people do not receive proper treatment, the urgency for proper accessible diagnosis and treatment is increasing.
WatchPAT™ is sold at 780,000 yen (excluding tax), or is leased for 22,000 yen per month (excluding tax) to medical institutions in Japan.
About Philips Respironics GK
Founded in 1984, Philips Respironics GK develops and imports high technology ME equipment and is dedicated to respiratory and sleeping medicine. The company also imports high-performance ventilator, CPAP and associated equipment.
For more information about Philips Respironics GK, please visit the official Web-site (http://www.respironics.philips.co.jp).
About Philips in Japan
Nihon Denshi Kaihatu Kabushikigaisha (currently Philips Electronics Japan) started importing Philips products to the Japanese market in 1953. In 2005, the company changed its name to Philips Electronics Japan. Currently, the company specializes in Healthcare, Lighting and Consumer Lifestyle.
In 2008, Philips Electronics Japan took over Fuji Respironics and in 2010 the company changed its name to Philips Respironics GK. Philips Electronics Japan employs about 1,700 employees including employees at Philips Respironics and deploys about 80 offices nationwide. For more details, please refer to the company’s web site. (http://www.philips.co.jp)
About Royal Philips:
Royal Philips (NYSE: PHG, AEX: PHIA) is a diversified health and well-being company, focused on improving people’s lives through meaningful innovation in the areas of Healthcare, Consumer Lifestyle and Lighting. Headquartered in the Netherlands, in 2013 Philips reported sales of EUR 23.3 billion and employs approximately 115,000 employees with sales and services in more than 100 countries. The company is a leader in cardiac care, acute care, home healthcare, energy efficient lighting solutions and new lighting applications, as well as male shaving and grooming and oral healthcare. News about Philips can be found at www.philips.com/newscenter.
About Itamar Medical
Itamar Medical, established in 1997 and based in Israel (traded on Tel Aviv stock exchange: ITMR), is a medical device company that develops, markets and sells diagnostic medical devices based on the PAT® (Peripheral Arterial Tone) signal. The PAT® signal is a non-invasive "window" to the cardiovascular system and the autonomic nervous system.
The company’s WatchPAT™ is a medical-grade portable diagnostic device that uses the most innovative technology to ensure accurate screening, detection, and assessment of sleep apnea. An additional device, the EndoPAT™ is an innovative diagnostic tool that provides doctors and patients a window to the current function of the endothelium and the overall health of the heart. The non-invasive diagnostic tool is indicated for use in endothelial function assessment.
Forward-looking statements
This release may contain certain forward-looking statements with respect to the financial conditions, results of operations and business of Philips and certainties of the plans and objectives of Philips with respect to these items.