MONMOUTH JUNCTION, N.J.--(BUSINESS WIRE)--Insmed Incorporated (Nasdaq GS:INSM), a biopharmaceutical company focused on developing an inhaled anti-infective to treat patients battling serious lung diseases in orphan indications that are often life-threatening, provides an interim update from the Company’s CLEAR-110 study, an ongoing, two-year, open-label extension study of once-daily ARIKACE®, or liposomal amikacin for inhalation, to treat Pseudomonas aeruginosa (Pa) in cystic fibrosis (CF) patients. These data are from 98 patients who have completed the first 12 months (six cycles) of the CLEAR-110 extension study. The data were collected as part of a scheduled data safety monitoring board (DSMB) review of the CLEAR-110 extension study. The data showed that ARIKACE was well tolerated, and there was a sustained improvement from baseline level in Forced Expiratory Volume in One Second (FEV1). These patients also experienced a sustained reduction in density of Pa sputum while on treatment.
The Two-Year Open-label Extension Study
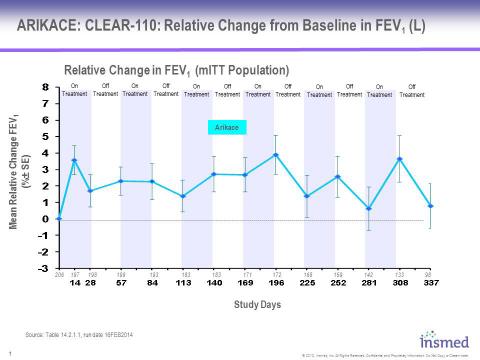
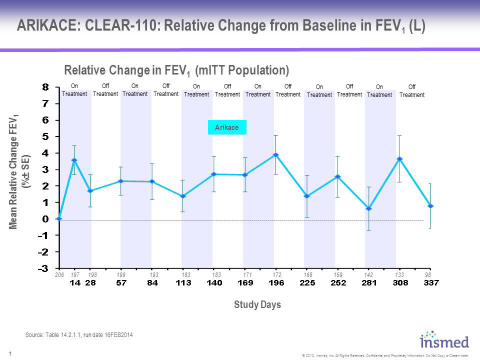
Eligible patients from the open-label, multicenter, randomized Phase 3 CLEAR-108 trial (as described below) were given the option to participate in a two-year, open-label, multi-cycle extension study designed to evaluate the long-term safety and tolerability of ARIKACE in CF patients. 206 patients enrolled in this study, representing 77% of the patients that completed the randomized portion of the Phase 3 trial, and received at least one dose of study drug. The data presented in the attached charts and discussed below includes 98 patients who had completed one year in the CLEAR-110 study by December 31, 2013, the time of data cut-off for DSMB review.
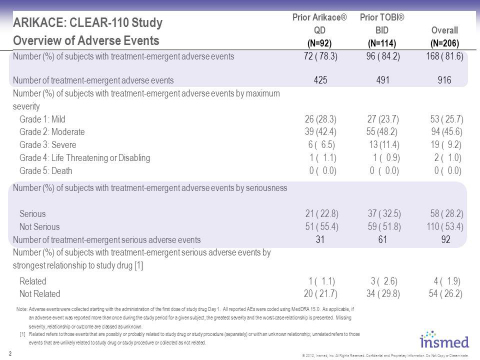
These data demonstrate that patients receiving ARIKACE for six cycles (12 months) in the extension study showed mean increase in relative change in FEV1 which is sustained during both on-treatment and off-treatment months. Overall, ARIKACE was well-tolerated during the six cycles administered over this 12-month period, with adverse events being consistent with those expected in a population of CF patients receiving inhaled medicines.
"As we announced on July 1, 2013, the Phase 3 study of ARIKACE achieved its primary endpoint and demonstrated that ARIKACE administered once a day is non-inferior to the standard of care which is administered twice a day. This longer-term study demonstrates our continued commitment to the CF patients and to gathering longer term data to clarify the safety and efficacy profile of our drug candidate. The data we are reporting today are consistent with the findings from our earlier longer term study of ARIKACE and we believe further strengthen the clinical data packages we plan to submit to European and Canadian regulatory authorities later this year," said Renu Gupta, MD, FAAP, Executive Vice President Development and Chief Medical Officer of Insmed.
“We are especially pleased that these data demonstrated that patients taking ARIKACE actually saw their FEV1 levels remain above baseline on average. In addition we continue to see reduction of bacterial density and consistency in our overall safety profile. Further, we believe that once-daily administration of ARIKACE will support patient convenience and compliance,” said Will Lewis, President and Chief Executive Officer of Insmed. “With the results achieved in our Phase 3 trial, along with these positive interim data, we continue our preparation for regulatory filings with the European Medicines Agency and Health Canada, which remain on target for mid-year.”
“I would like to recognize Dr. Gupta and our clinical team for designing and conducting such an important trial. This is the first trial to investigate the safety and efficacy of inhaled antibiotics in CF patients for a period of up to two years and it speaks to Insmed’s ongoing commitment to understanding the safe and effective use of this drug in this target population,” added Mr. Lewis.
Recap of ARIKACE Data from Phase 3 Clinical Trial to Treat Pa in CF Patients
The Phase 3 trial was designed to assess the comparative safety and efficacy of ARIKACE and TOBI in CF patients with Pa. A total of 302 adult and pediatric CF patients with chronic Pa were enrolled at 75 sites in Europe and Canada, and randomized to receive 28 days of ARIKACE treatment delivered once-daily via an investigational eFlow® Nebulizer System, or TOBI delivered twice-daily via the PARI LC Plus® Nebulizer System over a 24-week treatment period.
The primary endpoint was relative change in FEV1 measured after three treatment cycles, with each cycle consisting of 28 days “on” treatment and 28 days “off” treatment. Secondary endpoints measured were relative changes in FEV1 at other time points, time to and number of pulmonary exacerbations, time to antibiotic rescue treatment, change in density of Pa in sputum, respiratory hospitalizations and changes in Patient Reported Outcomes assessing Quality of Life.
The Phase 3 clinical trial of ARIKACE achieved its primary endpoint of non-inferiority to TOBI for relative change in FEV1 from baseline to end of study. Overall, the secondary endpoints showed comparability of once-daily ARIKACE compared with twice-daily TOBI consistent with the primary endpoint of the study. The overall safety profile of ARIKACE was comparable to TOBI, with adverse events consistent with those seen in similar studies and expected in a population of CF patients receiving inhaled antibiotics. There was no difference between arms in the reporting of serious adverse events and there were no unexpected adverse events.
About Cystic Fibrosis
CF is an inherited chronic disease that affects roughly 70,000 children and adults worldwide, including 30,000 children and adults in the US (Cystic Fibrosis Foundation Patient Registry, 2011) and 35,000 patients in Europe (Hoiby, BMC Medicine, 2011, 9:32). In CF patients, a defective gene and its protein product cause the body to produce unusually thick, sticky mucus that clogs the lungs and leads to life-threatening lung infections and obstructs the pancreas and stops natural enzymes from helping the body break down and absorb food. There is no cure for CF.
About ARIKACE®
ARIKACE is a form of the antibiotic amikacin, which is enclosed in nanocapsules of lipid called liposomes. This advanced pulmonary liposome technology prolongs the release of amikacin in the lungs while minimizing systemic exposure. The treatment uses biocompatible lipids endogenous to the lung that are formulated into small (0.3 micron), charge-neutral liposomes. ARIKACE is administered once-daily using an optimized, investigational eFlow® Nebulizer System manufactured by PARI Pharma GmbH, a novel, highly efficient and portable aerosol delivery system.
ARIKACE has been granted orphan drug designation in the U.S. by the FDA for the treatment of Pseudomonas infections in patients with CF and for the treatment of NTM lung infections. ARIKACE has also received orphan drug designation in Europe by the EMA for the treatment of Pseudomonas infections in patients with CF.
About eFlow® Technology and PARI Pharma
ARIKACE is delivered by an investigational eFlow® Nebulizer System developed by PARI Pharma and optimized specifically for ARIKACE. The optimized device uses eFlow Technology to enable highly efficient aerosolization of medication including liposomal formulations via a vibrating, perforated membrane that includes thousands of laser-drilled holes. Compared with other nebulization technologies, eFlow Technology produces aerosols with a very high density of active drug, a precisely defined droplet size and a high proportion of respirable droplets delivered in the shortest possible period of time. eFlow Technology is not an ultrasonic nebulizer technology and is not a general purpose electronic aerosol generator nebulizer technology. Combined with its quiet mode of operation, small size, light weight and battery use, eFlow Technology reduces the burden of taking daily, inhaled treatments. PARI Pharma focuses on the development of aerosol delivery devices and inhalation drug development to advance aerosol therapies where drug and device can be optimized together. For more information, please visit www.paripharma.com.
* TOBI® is a Registered Trademark of Novartis Pharmaceuticals Corporation.
About Insmed
Insmed Incorporated is a biopharmaceutical company dedicated to improving the lives of patients battling serious lung diseases. Insmed is focused on the development and commercialization of ARIKACE®, or liposomal amikacin for inhalation, for at least two identified orphan patient populations: patients with non-tuberculous mycobacteria (NTM) lung infections and cystic fibrosis (CF) patients with Pseudomonas aeruginosa lung infections. For more information, please visit www.insmed.com.
Forward-Looking Statements
This release contains forward-looking statements that are made pursuant to provisions of Section 21E of the Securities Exchange Act of 1934. Words, and variations of words, such as "intend," "expect," "will," "anticipate," "believe," "continue," "propose" and similar expressions are intended to identify forward-looking statements. Investors are cautioned that such statements in this release, including statements relating to the status, results and timing of clinical trials and clinical data, the anticipated benefits of Insmed's products, the anticipated timing of regulatory submissions, and the ability to obtain required regulatory approvals, bring products to market and successfully commercialize products constitute forward-looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, without limitation, failure or delay of European, Canadian, U.S. Food and Drug Administration and other regulatory reviews and approvals, competitive developments affecting the Company’s product candidates, delays in product development or clinical trials or other studies, patent disputes and other intellectual property developments relating to the Company’s product candidates, unexpected regulatory actions, delays or requests, the failure of clinical trials or other studies or results of clinical trials or other studies that do not meet expectations, the fact that subsequent analyses of clinical trial or study data may lead to different (including less favorable) interpretations of trial or study results or may identify important implications of a trial or study that are not reflected in the statements contained in this press release, and the fact that trial or study results or subsequent analyses may be subject to differing interpretations by regulatory agencies, the inability to successfully develop the Company’s product candidates or receive necessary regulatory approvals, inability to make product candidates commercially successful, changes in anticipated expenses, changes in the Company’s financing requirements or ability raise additional capital, and other risks and challenges detailed in the Company’s filings with the U.S. Securities and Exchange Commission, including its Annual Report on Form 10-K for the year ended December 31, 2012. Investors are cautioned not to place undue reliance on any forward-looking statements that speak only as of the date of this news release. The Company undertakes no obligation to update these forward-looking statements to reflect events or circumstances or changes in its expectations.