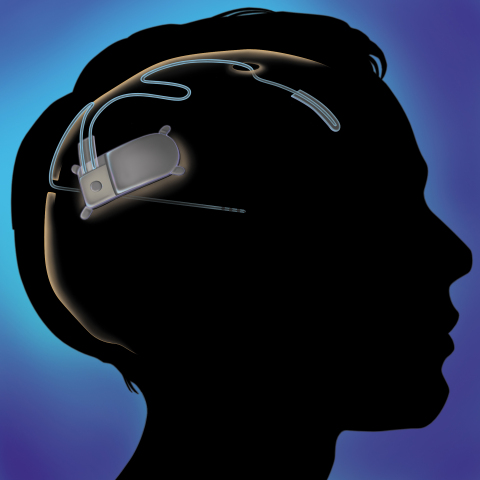
MOUNTAIN VIEW, Calif.--(BUSINESS WIRE)--NeuroPace, Inc. today announced that the U.S. Food and Drug Administration has granted premarket approval for the NeuroPace® RNS® System, a treatment for adults with partial onset seizures that have not been controlled with two or more antiepileptic drugs. The RNS System is a novel, implantable therapeutic device that delivers responsive neurostimulation, an advanced technology designed to detect abnormal electrical activity in the brain and respond by delivering imperceptible levels of electrical stimulation to normalize brain activity before an individual experiences seizures.
“We believe the RNS System has the potential to provide substantial improvement in quality of life to hundreds of thousands of people diagnosed with epilepsy in the U.S. who are unable to achieve seizure control with medications,” said Frank Fischer, NeuroPace CEO. “We anticipate that physicians will be able to make this breakthrough therapy available to eligible patients in the very near future.”
The RNS System is indicated for use as an adjunctive therapy in reducing the frequency of seizures in individuals 18 years of age or older with partial onset seizures who have undergone diagnostic testing that localized no more than two epileptogenic foci, are refractory to two or more antiepileptic medications, and currently have frequent and disabling seizures (motor partial seizures, complex partial seizures and/or secondarily generalized seizures). The RNS System has demonstrated safety and effectiveness in patients who average three or more disabling seizures per month over the three most recent months (with no month with fewer than two seizures), and has not been evaluated in patients with less frequent seizures. It is estimated that approximately 400,000 people in the U.S. meet these criteria and may benefit from treatment with the RNS System.
“The Epilepsy Foundation celebrates PMA approval of the RNS System,” said Phil Gattone, CEO of the Epilepsy Foundation. “We badly need new, effective therapies for the hundreds of thousands of people in this country as well as the millions around the world who live with uncontrolled seizures,” added Warren Lammert, Chairman of the Epilepsy Foundation. “The RNS System from NeuroPace integrates the best of technology and neurology, and is an important new treatment option for these individuals and their families.”
The RNS System has been evaluated in three clinical trials, including a prospective, randomized, double-blinded, sham stimulation controlled pivotal study. The pivotal study primary effectiveness endpoint was met by demonstrating a 37.9 percent reduction in seizure frequency in patients treated with responsive stimulation compared to a 17.3 percent reduction in patients who were implanted with the device but were not receiving responsive stimulation during a three month blinded period. The difference is statistically significant (p=0.012). For those subjects who reached two years post-implant, 55 percent of the subjects experienced a 50 percent or greater reduction in seizures.
“The clinical community is eager for a new therapeutic option for patients who continue to suffer the devastating consequences of uncontrolled partial seizures,” said Martha Morrell, MD, NeuroPace Chief Medical Officer and Clinical Professor of Neurology at Stanford University. “The results of the pivotal study clearly demonstrate that the safety and efficacy of the RNS System is sustained over two years. Additional data about safety and efficacy beyond two years is being collected in a long-term follow-up study.”
The pivotal trial primary safety endpoint was met by demonstrating a serious adverse event rate for the first 4 and 12 weeks post-implant comparable to similar procedures. There was no difference between the active and sham stimulation groups in the rate of adverse events, including depression, memory impairment and anxiety. There were no serious unanticipated device related adverse events reported in any of the clinical trials. Although there can be no assurances that additional long-term data will not reveal new adverse information presently unknown to NeuroPace, two year data shows no increase or worsening of adverse events.
A total of 256 patients have been implanted with the RNS System. At this time, some patients have been treated with the RNS System for over eight years, and more than 1,200 patient years of experience with responsive neurostimulation have been accumulated to date.
About the RNS® System
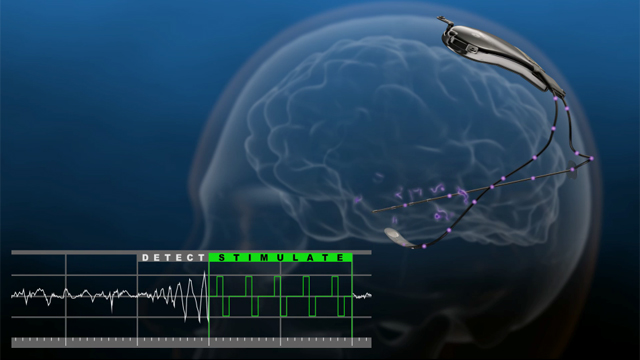
The RNS System is the first closed-loop responsive brain stimulation system. The system is designed to treat partial onset seizures by detecting specific types of electrical activity in the brain through leads containing electrodes that are placed near the patient’s seizure focus or foci. When detection thresholds are met, the device delivers small bursts of electrical stimulation intended to reduce the frequency of seizures. Physicians can program the detection and stimulation parameters of the implanted RNS Neurostimulator non-invasively to customize therapy for each individual.
About the RNS® System Pivotal Clinical Trial
Trial Patient Population
The RNS System Pivotal Clinical Investigation was a prospective, randomized, double-blind, sham stimulation controlled investigation that included 191 people implanted with the RNS System across 32 Comprehensive Epilepsy Centers. All subjects in the study were required to be 18-70 years of age and have partial onset epilepsy (with seizures that start from one or two areas of the brain) that had not been effectively treated with two or more antiepileptic medications.
Trial Design
Patients in the study were implanted with the RNS® Neurostimulator and leads once eligibility criteria were met. The blinded evaluation period of the trial began eight weeks after implant and lasted 12 weeks. Half the participants were randomly assigned to have responsive stimulation activated and half had responsive stimulation remain inactive. Participants and one doctor (assessment physician) at each site in the trial did not know whether stimulation was active or not. A separate doctor (treatment physician) at each site programmed the devices in order to ensure that the assessment physician remained blinded. Five months after implantation, when the double-blinded portion of the trial was completed, stimulation was activated for all participants in the trial. Each participant in the trial was evaluated for two years following implant. After completion of the pivotal trial, all patients were given the option of enrolling in the RNS® System Long-term Treatment Clinical Investigation designed to provide up to an additional seven years of safety and effectiveness data.
About NeuroPace
NeuroPace designs, develops, manufactures and intends to market implantable devices for the treatment of neurological disorders. The company’s initial focus is the treatment of epilepsy, a debilitating neurological disorder affecting approximately one percent of the population worldwide. An estimated 30-40 percent of the 65 million people worldwide (including nearly three million Americans) with epilepsy experience uncontrolled seizures. In addition to treating epilepsy, responsive neurostimulation holds the promise of treating several other disabling neurological disorders that negatively impact quality of life for millions of patients throughout the world.
Located in Mountain View, California, NeuroPace is a privately held company.